Abstract
Objective
To evaluate the prophylactic use of enteral fluconazole to prevent invasive candidal infections in critically ill surgical patients.
Summary Background Data
Invasive fungal infections are increasingly common in the critically ill, especially in surgical patients. Although fungal prophylaxis has been proven effective in certain high-risk patients such as bone marrow transplant patients, few studies have focused on surgical patients and prevention of fungal infection.
Methods
The authors conducted a prospective, randomized, placebo-controlled trial in a single-center, tertiary care surgical intensive care unit (ICU). A total of 260 critically ill surgical patients with a length of ICU stay of at least 3 days were randomly assigned to receive either enteral fluconazole 400 mg or placebo per day during their stay in the surgical ICU at Johns Hopkins Hospital.
Results
The primary end point was the time to occurrence of fungal infection during the surgical ICU stay, with planned secondary analysis of patients “on-therapy” and alternate definitions of fungal infections. In a time-to-event analysis, the risk of candidal infection in patients receiving fluconazole was significantly less than the risk in patients receiving placebo. After adjusting for potentially confounding effects of the Acute Physiology and Chronic Health Evaluation (APACHE) III score, days to first dose, and fungal colonization at enrollment, the risk of fungal infection was reduced by 55% in the fluconazole group. No difference in death rate was observed between patients receiving fluconazole and those receiving placebo.
Conclusions
Enteral fluconazole safely and effectively decreased the incidence of fungal infections in high-risk, critically ill surgical patients.
Fungal pathogens increasingly cause nosocomial infections, especially among surgical patients and the high-risk critically ill, with an attributable death rate estimated at 38%. 1–9 In our institution, yeast species accounted for 15% of bloodstream isolates in the period from 1992 to 1995. 10
Fluconazole is a triazole antifungal drug with excellent enteral bioavailability, low toxicity, and activity against many pathogenic Candida species. 11,12 Fluconazole has been shown to prevent both deep fungal infections in bone marrow transplant populations 13 and superficial fungal infections in patients with leukemia. 14 The role for the empiric use of fluconazole in intensive care unit (ICU) patients, however, remains controversial. 15–17
Given the high incidence of candidal infection among critically ill surgical patients, we hypothesized that these infections could be prevented in high-risk patients by using prophylactic enteral fluconazole.
PATIENTS AND METHODS
Patients
Patients enrolled in this trial had to have an expected length of ICU stay of 3 or more days as determined by an experienced intensivist (P.A.L.), based on admitting diagnosis, magnitude of hemodynamic perturbation, ventilatory failure, and baseline medical conditions. Patients were excluded for the following reasons: pregnancy, receipt of antifungal agents within the 7 days before ICU admission, age younger than 18, or an expectation that the patient would not survive more than 24 hours. The study was conducted between January 7, 1998, and January 13, 1999. The study protocol was approved by the Institutional Review Board at Johns Hopkins Hospital. Informed consent was obtained from all patients or a legal proxy before enrollment.
Randomization
After enrollment, individual patients were randomly assigned by block design by the hospital pharmacy to receive, that day, a single, daily, enteral dose of fluconazole suspension or identical placebo. All patients and investigators were masked to the treatment assignments.
Intervention
The loading dose was 800 mg, followed by a 400-mg daily dose. If the estimated creatinine clearance was less than 25 mL/min, the daily dose was reduced to 200 mg. No intravenous preparation of the study drug was given. Administration of the study drug continued until initiation of empiric antifungals or ICU discharge. Patients were deemed to have completed the study at the time of ICU death, institution of systemic antifungal therapy, or 3 days after ICU discharge. Decisions to initiate empiric antifungal treatment were made by nonstudy clinicians unaware of the study treatment and were based on clinical assessment and cultures.
To determine colonization and not infection, on the days of enrollment and discharge and every Monday and Thursday during the ICU stay, surveillance fungal cultures were obtained, where possible, from the oropharynx, endotracheal tube, rectum, ostomy drainage, bile, urine, and gastric aspirate. This information was not available to clinicians managing patients.
Definition of Events
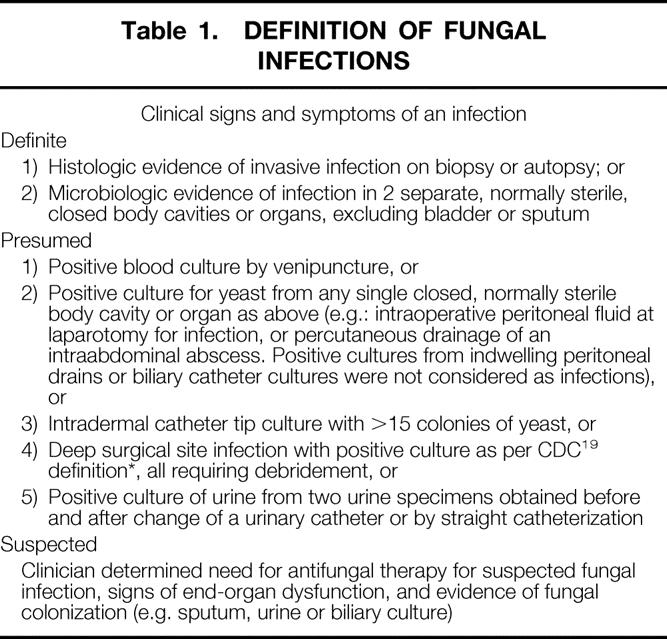
Events were classified using predetermined criteria by a masked adjudication panel. Fungal infections were classified as proven or suspected as shown in Table 1. These definitions are substantially consistent with the recently proposed consensus of the European and NIH groups for fungal infections. 18,19
Table 1. DEFINITION OF FUNGAL INFECTIONS
Statistical Analysis
All patients were included in the statistical analysis. Differences in proportions of baseline characteristics between the two treatment groups were assessed using the chi-square statistics. Distributions of continuous variables were compared using the Kruskal-Wallis test. The main analysis of this study was an intent-to-treat analysis of the time to the primary outcome of proven fungal infection. We calculated a sample size of 130 patients in each treatment group, based on a rate of fungal infections of 15% previously observed in a pilot study and an expected 4:1 ratio of fungal infections in the two treatment arms. The power was set at 80% and the two-sided significance level set at 0.05, and the sample size was inflated by 10% to account for an ineligibility rate of 10%.
In the main intent-to-treat analysis, time on study began when a patient was admitted to the surgical ICU and was randomized. Time on study ended with death, initiation of antifungal therapy, diagnosis of a fungal infection, or 3 days after ICU discharge. In a planned secondary “on-therapy” time to event analysis, time on study began the day after the patient received the loading dose of study drug and ended as above. Patients were excluded from the on-therapy analysis if they died, received empiric antifungal therapy, or were diagnosed with a fungal infection on or before the date on which the first dose of study drug was given. Secondary analyses were also conducted for alternate definitions of infections.
We used the log-rank test to compare the distribution of times to events in the treatment and control groups. 20 Proportions of patients developing infections over time were computed using Kaplan-Meier estimates. 21 Continuous dependent variables with nonnormal distributions were compared using the Kruskal-Wallis test. In all analyses, a two-sided probability value of 0.05 was considered to be statistically significant. The Cox proportional hazards model was used for multivariate analysis of predictors of fungal infection. 22 A linear random effects model was used in the longitudinal analysis of colonization with non-Candida albicans species of yeast within individuals over time on study. 23 Epi Info (Center for Disease Control, Atlanta, GA) and the Stata statistical package (College Station, TX) were used for all analyses.
RESULTS
Enrollment and Baseline Characteristics
Most patients who met eligibility criteria for the study were identified and approached for consent (Fig. 1). Of 1,228 surgical ICU admissions during this year-long study, 767 (62%) were ineligible for enrollment. Of 461 patients eligible for the study, 260 (56%) were enrolled in the study. Of the 260 patients in the intent-to-treat analysis, 130 received fluconazole and 130 received placebo.
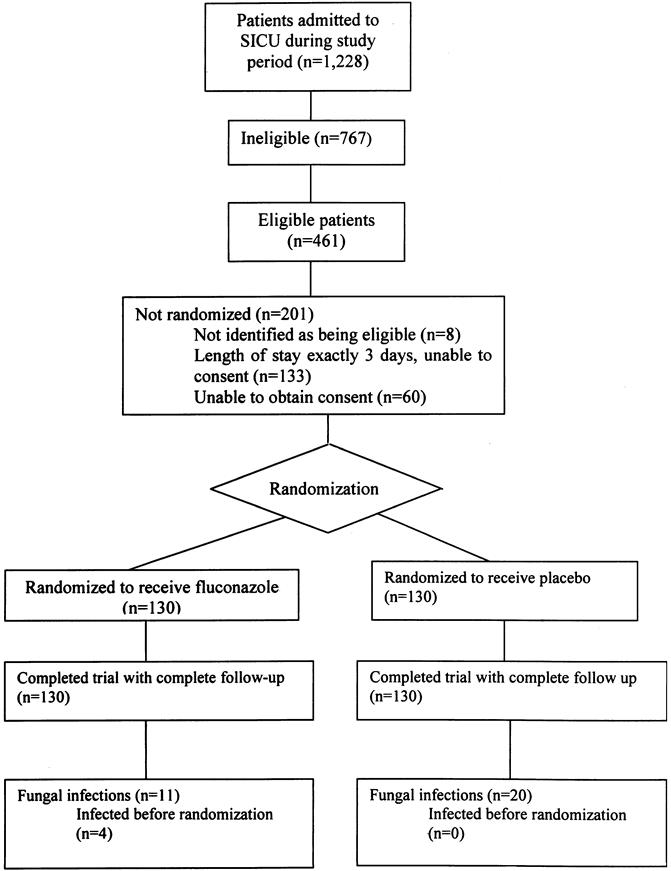
Figure 1. Enrollment of study cohort.
Twenty-two patients were excluded from the on-therapy analysis. Eleven patients did not meet the enrollment criteria (age younger than 18 years, consent withdrawn, antifungal use, length of stay more than 3 days). In addition, four of these patients had proven fungal infection on the basis of cultures taken before enrollment, but not reported as positive until after enrollment. Four patients never received a dose of the study drug, and three patients either died or were discharged on the day of the first dose. Of the 238 patients in the on-therapy analysis, 117 received fluconazole and 121 received placebo.
Baseline characteristics of the patients in the two groups were similar (Table 2). In general, they were critically ill, with multiple underlying medical conditions and high APACHE III scores.
Table 2. BASELINE CHARACTERISTICS OF STUDY COHORT

* Includes corticosteroids, cyclosporine, azathioprine, tacrolimus, and mycophenolate.
† Based on admitting physician’s assessment.
Fungal Infections
In the intent-to-treat analysis, a total of 31 patients had proven fungal infections during the study period, comprising 7 definite infections and 24 presumed infections Table 3). Eleven proven infections occurred in the fluconazole group and 20 in the placebo group. The most frequently infected site was the abdomen. In the intent-to-treat analysis, the absolute reduction in risk for fungal infection was 7% and the number needed to treat to prevent one fungal infection was 15. In the on-therapy analysis, which excluded the four patients with proven fungal infection diagnosed before randomization, seven infections were diagnosed in the fluconazole group and 20 in the placebo group. In the on-therapy analysis, the number needed to treat to prevent one fungal infection was 9.5.
Table 3. SITES OF PROVEN FUNGAL INFECTIONS
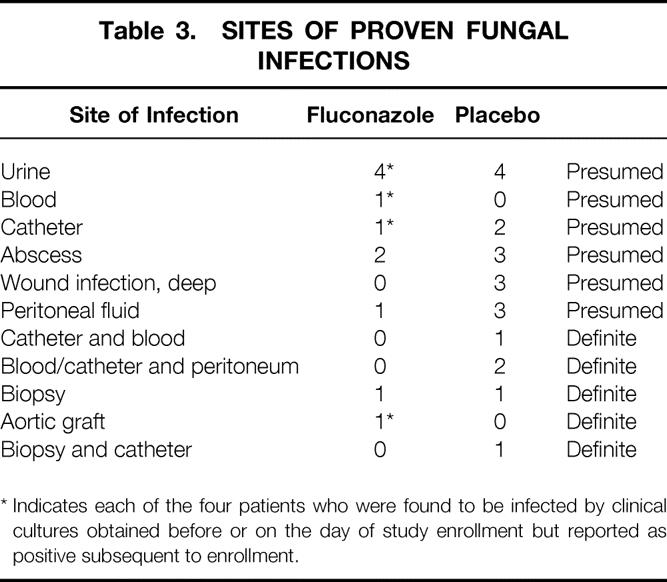
* Indicates each of the four patients who were found to be infected by clinical cultures obtained before or on the day of study enrollment but reported as positive subsequent to enrollment.
If patients with urinary tract infections were excluded from the proven infections, there were 7 infections in the fluconazole group and 16 in the placebo group. Similarly, exclusion of the central line catheters also maintained a significant difference in the two patient groups (P < .01). Eight additional patients met our definition of a suspected fungal infection, having been removed from the study for treatment with antifungals. Two of these suspected infections occurred in the fluconazole group and six in the placebo group.
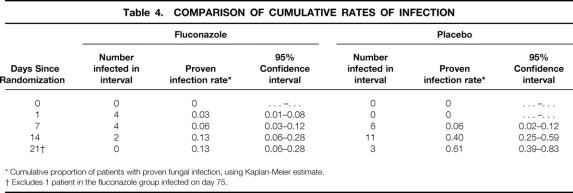
Time to event analyses comparing the fluconazole and placebo groups showed a marked benefit in patients receiving fluconazole (P = .01, log-rank test). Figure 2 shows the Kaplan-Meier curves comparing the treatment and control groups, and Table 4 shows the Kaplan-Meier analysis of the proportion of patients infected over time in the intent-to-treat analysis. The effect of fluconazole in preventing fungal infection was greater and more statistically significant if urinary tract infections were excluded, if suspected infections were included, or if the analysis was restricted to the on-therapy group (P < .01 in all analyses).

Figure 2. Kaplan-Meier curves showing time to proven infection, intent-to-treat analysis.
Table 4. COMPARISON OF CUMULATIVE RATES OF INFECTION
* Cumulative proportion of patients with proven fungal infection, using Kaplan-Meier estimate.
† Excludes 1 patient in the fluconazole group infected on day 75.
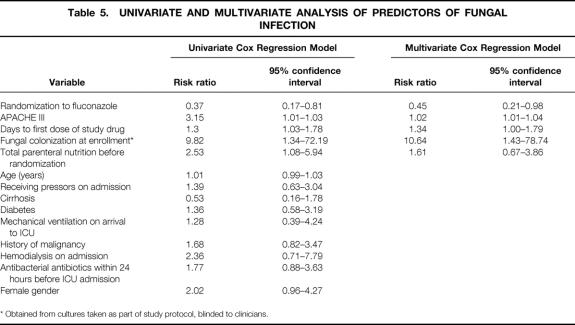
In a univariate time to event analysis, the variables having a statistically significant association with increased risk for fungal infections were randomization to placebo, time from ICU admission to first dose of study drug, APACHE III score, use of total parenteral nutrition before randomization, and fungal colonization at enrollment (Table 5). When these variables were all included in a Cox proportional hazard model, all remained statistically significant except for the use of total parenteral nutrition. The adjusted overall risk of fungal infection was reduced by 55% in the fluconazole group compared with the placebo group (relative risk 0.45, 95% confidence interval 0.21–0.98).
Table 5. UNIVARIATE AND MULTIVARIATE ANALYSIS OF PREDICTORS OF FUNGAL INFECTION
* Obtained from cultures taken as part of study protocol, blinded to clinicians.
The median length of stay in both the fluconazole and placebo groups was 5 days (range <1–123 in the fluconazole group, <1–69 in the placebo group;P = .8).
Microbiology
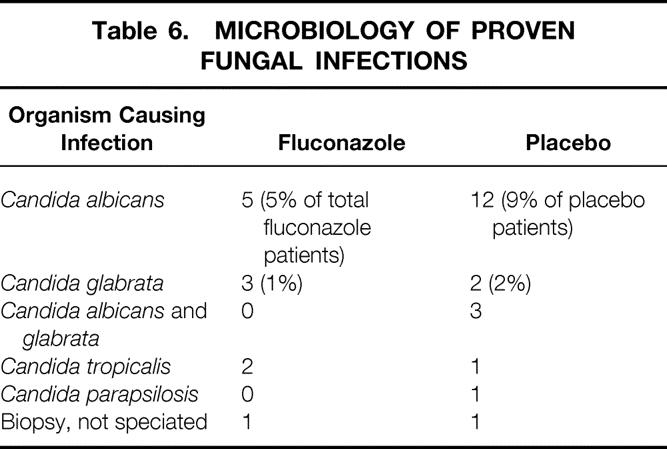
The most common species of yeast causing infection in this cohort was C. albicans (Table 6). Using a linear regression model adjusted for clustering on individual patients, we found no association between fluconazole minimum inhibitory concentration (MICs) and the use of fluconazole (P = .45). No change in the MICs of the infecting isolates was observed as a function of the duration of the study. Fluconazole use was not associated with a shift toward non-C. albicans species, based on two analyses. First, a chi-square analysis showed no association between receiving fluconazole and non-C. albicans species colonization (P = .42). Second, a longitudinal data analysis using a random effects model showed no association between receiving fluconazole and a shift towards non-C. albicans species in individual patients over the course of the ICU stay (P = .21).
Table 6. MICROBIOLOGY OF PROVEN FUNGAL INFECTIONS
Death
Thirty of 260 eligible patients (12%) died during their ICU stay, 14 in the fluconazole group and 16 in the placebo group. The Cox analysis showed no statistically significant difference in survival by treatment arm (relative risk 0.73, 95% confidence interval 0.41–1.32).
DISCUSSION
In this double-blind, placebo-controlled clinical trial, we have shown that prophylactic fluconazole prevents invasive candidal infections in critically ill surgical patients, independent of other risk factors for fungal infection. The efficacy of fluconazole in preventing fungal infection was evident in our conservative intent-to-treat analysis and was even more striking in all of our planned secondary analyses. Patients receiving fluconazole were not more likely to be colonized or infected with fluconazole-resistant Candida species. We did not expect to show a difference in death rates between the two groups because the study was sized to show a difference in infection rates.
Prior studies have shown fluconazole to be effective in preventing invasive fungal infections in bone marrow transplant and leukemia patients. 13,14 In two small studies of surgical patients, patients receiving prophylactic ketoconazole or fluconazole showed a decrease in fungal infections. 16,17 However, the use of prophylactic antifungals in the critically ill has not gained broad acceptance.
Our expectation was that patients with the greatest degree of critical illness and a prolonged ICU stay would be at the highest risk for fungal infection. Although this assessment was based on nonstandardized clinical evaluation, our ability to predict which patients would remain in the ICU with a critical illness at least 3 days was highly reliable. More than 90% of all eligible patients were correctly identified for study enrollment. We would expect that most clinicians with expertise in the care of critically ill patients will be able to predict which patients will require intensive care for at least 3 days with similar accuracy.
Patients requiring a 3-day length of stay in the surgical ICU of a tertiary referral center such as the Johns Hopkins Hospital may be more critically ill than patients with a comparable length of stay in other hospitals. This may cause difficulty in selecting patients who may benefit from this strategy at other institutions. The generalizability of these findings depends on the prevalence of fungal infections in an individual hospital and on clinicians’ ability to select prospectively patients at high risk for fungal infection. Patients with clinical characteristics similar to those described in this study (see Table 2) would be expected to be at high risk for fungal infection.
We chose to use enteral rather than intravenous fluconazole because it costs less than the intravenous preparation and appears to have adequate enteral bioavailability. 24–26 The pharmacokinetics of enteral fluconazole in this population requires further investigation. However, the efficacy of fluconazole in this study suggests that the enteral route is adequate for the present indication, and that the use of intravenous fluconazole should not be necessary.
Given the low cost of enteral fluconazole, the low number of patients needed to treat to prevent fungal infection in this study, and the high cost attributable to nosocomial infection, 27 the use of prophylactic fluconazole to prevent fungal infections in this population is likely to be cost-effective. Previously, we reported that the attributable increase in the cost of ICU care for patients with fungal infections is $21,590. 28 Given that fluconazole suspension costs roughly $90 for a 400-mg dose (average wholesale price), and that the median length of ICU stay in this cohort was 5 days, it is likely that the cost of fluconazole prophylaxis will be substantially less than the attributable cost of nosocomial fungal infections. Further analysis will be required to assess the cost-effectiveness of the use of fluconazole prophylaxis in this population.
Although the definition of fungal infections remains controversial, we believe that the infection definitions used in this study were conservative and reflect common and appropriate clinical definitions. The most controversial definitions for fungal infection were the inclusion of catheters with more than 15 colonies, and urinary tract infections. Isolation of Candida species from the peritoneum was included as an infection definition because it has a high associated death rate, 29–31 and most experts would institute antifungal therapy in the setting of clinical peritonitis with positive cultures for Candida species. 15 We performed secondary analysis using these alternative infection definitions, and all findings remained significant. The rate of Candida infection of 16% among placebo recipients in the present study is lower than infection rates reported in other high-risk groups, 8,14,17 suggesting that our infection definitions are indeed conservative and consistent with the experience of other investigators.
The widespread use of fluconazole may lead to an increase in the isolation of Candida krusei32 and Candida glabrata, 33 two species of fungi that are often resistant to fluconazole. In the present study, we found no evidence of increased resistance to fluconazole among patients receiving fluconazole, nor was there an increase in the isolation of non-C. albicans species, either within individual patients or over the course of this 1-year study. Although it is possible that the widespread use of fluconazole to prevent fungal infections in the ICU may lead to a shift toward more fluconazole-resistant Candida isolates over greater time, we found no evidence of such a trend in the present study. In addition, the numbers of infections with C. glabrata were similar in the fluconazole and placebo groups. Despite the reported shift toward non-C. albicans species of fungi as human pathogens, most infecting Candida isolates remain sensitive to fluconazole. 8,34,35
Fungal infections are an increasingly common and serious problem in the critically ill. In this study, we showed that the use of prophylactic enteral fluconazole in the setting of a large university hospital in critically ill surgical patients with an expected length of stay of 3 or more days results in fewer definite or presumed fungal infections and a decreased risk of infection over time. Critically ill patients in other settings may similarly benefit from this therapy. Additional long-term epidemiologic data must be obtained to determine the effect on fungal resistance patterns.
Acknowledgments
The authors thank, for their support and cooperation, the nurses, technicians, and physician staff involved in the care of these patients in the Surgical Intensive Care Unit. The authors also thank Douglas Webb, PhD, for his dedication and interest in the conduct and completion of this project.
Footnotes
Pfizer Pharmaceuticals, Inc., provided fluconazole and an Unrestricted Educational Grant that was used to support the design, conduct, and analyses of this project independently by the authors at Johns Hopkins University.
Dr. Hammond is now employed by Glaxo Wellcome, Research Triangle Park, North Carolina.
Presented in abstract form at the Society of Critical Care Medicine meeting, Orlando, Florida, February 12–16, 2000.
Correspondence: Dr. Pamela A. Lipsett, 600 N. Wolfe St., Blalock 685/683, Baltimore, MD 21287-4605
E-mail: plipsett@jhmi.edu
Accepted for publication August 28, 2000.
References
- 1.Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 1995; 20: 1526–1530. [DOI] [PubMed] [Google Scholar]
- 2.Wey SB, Mori M, Pfaller MA, et al. Risk factors for hospital-acquired candidemia. Arch Intern Med 1989; 149: 2349–2353. [PubMed] [Google Scholar]
- 3.Karabinis A, Hill C, Leclerq B, et al. Risk factors for candidemia in cancer patients: a case-control study. J Clin Microbiol 1988; 26: 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bross J, Talbot GH, Maislin G, et al. Risk factors for nosocomial candidemia: a case-control study. Am J Med 1989; : 614–620. [DOI] [PubMed] [Google Scholar]
- 5.Komshian SV, Uwaydah A, Sobel JD, et al. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis 1989; 11: 379–390. [DOI] [PubMed] [Google Scholar]
- 6.Wey SB, Mori M, Pfaller MA, et al. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 1988; 148: 2642–2645. [DOI] [PubMed] [Google Scholar]
- 7.Harvey RL, Myers JP. Nosocomial fungemia in a large community teaching hospital. Arch Intern Med 1987; 147: 2117–2120. [PubMed] [Google Scholar]
- 8.Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1989–1989. Am J Med 1991(Suppl 3B): 86S–89S. [DOI] [PubMed]
- 9.Beck-Sague CM, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis 1993; 167: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 10.Maenza JR, Merz WG. Candidemia: epidemiology and laboratory detection. Infect Dis Clin Pract 1997; 6: 83–88. [Google Scholar]
- 11.Debruyne D, Ryckelynck J. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinetics 1993; 24 (1): 10–27. [DOI] [PubMed] [Google Scholar]
- 12.Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988; 32: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845–851. [DOI] [PubMed] [Google Scholar]
- 14.Winston DJ, Chandrasekar PH, Lazarus HM, et al. Fluconazole prophylaxis of fungal infections in patients with acute leukemia. Results of a randomized placebo-controlled, double blind multicenter trial. Ann Intern Med 1993; 118: 495–503. [DOI] [PubMed] [Google Scholar]
- 15.Edwards JE, Bodey LGP, Bowden RA, et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis 1997; 25: 43–59. [DOI] [PubMed] [Google Scholar]
- 16.Slotman GJ, Burchard KW. Ketoconazole prevents Candida sepsis in critically ill surgical patients. Arch Surg 1987; 122: 147–151. [DOI] [PubMed] [Google Scholar]
- 17.Eggimann P, Francioli P, Bille J, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med 1999; 27: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 18.Ascioglu S, De Pauw B, Bennett JE, et al. Analysis of Definitions Used in Clinical Research on Invasive Fungal Infections (IFI): Consensus Proposal for New, Standardized Definitions. 1999, A1639, ICACC (39th Interscience Conference on Antimicrobial Agents and Chemotherapy).
- 19.Centers for Disease Control & Prevention. Draft guidelines for the prevention of surgical site infection, 1998. Federal Register 1998; 63 (116): 33168–33192. [PubMed] [Google Scholar]
- 20.Peto R, Peto J. Asymptotically efficent rank invariant procedures. J R Stat Soc A 1972; 135 (pt 2):185–206. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J R Stat Soc B 1972; 134 (pt 2):187–220. [Google Scholar]
- 23.Diggle PJ, Liang KY, Zeger S. Analysis of longitudinal data. Oxford: Oxford University Press; 1998.
- 24.Nicolau DP, Crowe H, Nightingale CH, et al. Bioavailability of fluconazole administered via a feeding tube in intensive care unit patients. J Antimicrob Chemother 1995; 36: 395–401. [DOI] [PubMed] [Google Scholar]
- 25.Rosemurgy AS, Markowsky S, Goode SE, et al. Bioavailability of fluconazole in surgical intensive care unit patients: a study comparing routes of administration. J Trauma 1995; 39: 445–447. [DOI] [PubMed] [Google Scholar]
- 26.Joe LA, Jacobs RA, Guglielmo BJ. Systemic absorption of oral fluconazole after gastrointestinal resection. J Antimicrob Chemother 1994; 33: 1070. [DOI] [PubMed] [Google Scholar]
- 27.Pittet D, Tarara D, Wenzel R. Nosocomial bloodstream infection in critically ill patient. Excess length of stay, extra costs, and attributable mortality. JAMA 1994; 271: 1598–1601. [DOI] [PubMed] [Google Scholar]
- 28.Pelz R, Hendrix CW, Swoboda S, et al. Do fungal infections increase ICU cost? Abstract, 1999 meeting of the Surgical Infection Society.
- 29.Lecciones JA, Lee JW, Navarro EE, et al. Vascular catheter-associated fungemia in patients with cancer: analysis of 155 episodes. Clin Infect Dis 1992; 14: 875–883. [DOI] [PubMed] [Google Scholar]
- 30.Rex JH, et al. Intravascular catheter exchange and duration of candidemia. Clin Infect Dis 1995; 21: 994–996. [DOI] [PubMed] [Google Scholar]
- 31.Calandra T, Bille J, Schneider R, et al. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet 1989; 22: 1437–1440. [DOI] [PubMed] [Google Scholar]
- 32.Wingard JR, Merz WG, Rinaldi MG, et al. Increase in Candida krusei infection among patient with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med 1991; 325: 1274–1277. [DOI] [PubMed] [Google Scholar]
- 33.Wingard JR, Merz WG, Rinaldi MG, et al. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother 1993; 37: 1847–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingard JR. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis 1995; 20: 115–125. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller MA. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis 1996; 22: S89–94. [DOI] [PubMed] [Google Scholar]