Abstract
Objective
To identify the outcomes and risks of split-liver transplantation (SLT) for two adult recipients to determine the feasibility of more widespread use of this procedure to increase the graft pool for adults.
Summary Background Data
The shortage of cadaver liver grafts for adults is increasing. Using livers from donors defined as optimal, the authors have been developing techniques for SLT for two adult recipients at their center.
Methods
From July 1993 to December 1999, 34 adults have undergone SLT with grafts from optimal donors prepared by ex situ split (n = 30) or in situ split (n = 4), and 88 adults received optimal whole-liver grafts that were not split. Four split-grafts were transplanted at other centers. The outcomes of transplantation with right and left split-liver grafts were compared with those of whole-liver transplants. The main end points were patient and graft survival at 1 and 2 years and the incidence and types of complications.
Results
For whole-liver, right and left split-liver grafts, respectively, patient survival rates were 88%, 74%, and 88% at 1 year and 85%, 74%, and 64% at 2 years. Graft survival rates were 88%, 74%, and 75% at 1 year and 85%, 74%, and 43% at 2 years. Patient survival was adversely affected by graft steatosis and recipients inpatient status before transplantation. Graft survival was adversely affected by steatosis and a graft-to-recipient body weight ratio of less than 1%. Primary nonfunction occurred in three left split-liver grafts. The rates of arterial (6%) and biliary (22%) complications were similar to published data from conventional transplantation for an adult and a child. SLT for two adults increased the number of recipients by 62% compared with whole-liver transplantation and was logistically possible in 16 of the 104 (15%) optimal cadaver donors.
Conclusions
Split-liver transplantation for two adults is technically feasible. Outcomes and complication rates can be improved by rigid selection criteria for donors and recipients, particularly for the smaller left graft, and possibly also by in situ splitting in cadaver donors. Wider use will require changes in the procedures for graft allocation and coordination between centers experienced in the techniques.
The gap between the supply of livers for transplantation and the demand continues to widen as new indications are added, with adult potential recipients accounting for more than 95% of waiting list deaths. 1 The development of new guidelines for accepting potential recipients has been recommended to limit this proportion, ensuring that available organs go to patients whose condition has not deteriorated during the waiting time and who are more likely to enjoy longer-term survival. 2 However, we believe that there is still scope for increasing the graft pool for adults through split-liver transplantation (SLT).
Split-liver procedures that divide a cadaver organ into a small left graft for a child and a larger right graft for an adult have reduced the graft shortage for children 3 and could even eliminate the need for elective living donors in this population. 4,5 Split-liver procedures for two adult recipients, however, are still uncommon. They pose technical challenges, in particular that of obtaining an adequate mass of functional parenchyma in the left graft. Our earlier report on SLT 6 described three split-liver procedures for two adults. Since then, we have extended our experience using techniques such as ex situ splitting of cadaver donors and in situ splitting of livers from patients with familial amyloid polyneuropathy (FAP) undergoing domino liver transplantation. 7 In this article, we compare the outcomes of split-liver procedures for two adults with those of whole-liver transplantation from similar donors and discuss the technical and logistical feasibility, including criteria for donor and recipient selection.
PATIENTS AND METHODS
Organizational Aspects
The French Organ Sharing Authority (Etablissement Français des Greffes) allocates each donor to a center, which then selects the recipient. We consider as optimal for a split-liver procedure a donor younger than 55 years with body weight more than 70 kg who has stable hemodynamics, normal liver function tests, and no macroscopic aspect of liver steatosis at harvesting. 6 When a donor meets the preharvest criteria and local logistics permit the split-liver procedure, the French Organ Sharing Authority is informed and offers the potential second split graft to another center. The first recipient procedure starts as soon as the donor liver is assessed as suitable for transplantation. The anatomical feasibility of dividing the liver is confirmed on the back table using arteriography and cholangiography. 6 For FAP donors, arteriography is performed in the usual pretransplant workup and cholangiography is performed during surgery. 7,8 If another center has accepted a split-liver graft, the split graft to be shipped is prepared first and dispatched. If no other center has accepted the graft, a second local recipient is prepared when logistics permit. Otherwise, the liver graft is transplanted as a whole organ.
Between July 1993 and December 1999, there were 18 optimal donors whose liver was used in a split procedure for two adults undergoing a first liver transplantation. All recipients gave informed consent to receiving a split-liver graft. In the same period, livers from 88 optimal donors were used for first elective liver transplantation in whole-liver transplants because logistics did not allow the split procedure.
Split-Liver Graft Preparation
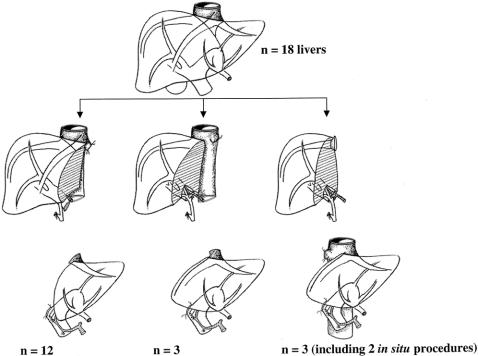
At the anatomical assessment, only nonbifurcation of the portal vein is an absolute barrier to splitting. Other vascular and biliary variants are not barriers, irrespective of the numbers and difficulty of the anastomoses required. In this series, the liver was divided through the middle of segment 4 in 12 of the 18 cadaver donors, retaining the middle hepatic vein with the right graft. 6 In six recent cases, all of segment 4 (the left medial segment) was allocated to the left graft to increase the graft-to-recipient body weight ratio. The middle hepatic vein was kept on the left in continuity with the common trunk of the left and middle hepatic veins. We used the cutting lines as for hepatectomy in living donors to remove the left liver 9 in three cases and for right liver removal 10 in the three others. In both FAP domino donors (the explanted native liver of a patient with FAP is split in situ and both explanted hemilivers are transplanted to two recipients), an in situ split procedure was performed 8,11 (Fig. 1).
Figure 1. Cutting lines used to split for two adults.
The first-order artery (celiac axis for cadaveric grafts, common hepatic artery for domino grafts) was retained on the left graft in 16 of the 18 grafts. The portal vein trunk was kept in continuity with the left graft when there was a portal bifurcation (15/18). In three cases with a trifurcation, the portal trunk was kept in continuity with the right graft.
Split-Liver Graft Implantation
The technique was selected as indicated for each split graft–patient couple 12–15 with percutaneous venovenous bypass installed as needed. 16 The implantation technique was adapted as necessary using preservation of the native vena cava, 17,18 arterial or venous grafts, 19–21 or the prevention of kinking of the venous anastomosis. 22 One patient underwent a flop procedure 23 because of the severe angulation of the vascular pedicle connections. In the so-called flop procedure, the left graft is rotated through 180° of sagittal orientation so that the hilar structures are brought into an anterior and medial location coaxial with the native liver pedicle. One patient underwent an auxiliary partial orthotopic liver transplantation for fulminant hepatitis. 24
First-order arteries were anastomosed with ×2.5 magnifying loupes, and second-order arteries were anastomosed under the microscope. 25,26
Postoperative Care
In all patients, the usual posttransplant care for the center was followed. 6 Graft perfusion was checked by Doppler ultrasound examination daily in the intensive care unit, then weekly until discharge from hospital. Patients whose partial thromboplastin time was less than 1.3 times control or whose platelet count was more than 30,000/μL were anticoagulated with heparin (target partial thromboplastin time 1.5–2.0 times control). Heparin was discontinued on discharge from the hospital, and aspirin (250 mg/day) was started.
Data Analysis
Continuous variables in recipients included age (years), body weight (kg), pretransplant levels of prothrombin (% of normal level), bilirubin (μmol/L), albumin (g/L), creatinine (μmol/L), alanine aminotransferase (ALAT) (IU/L), and aspartate aminotransferase (ASAT) (IU/L). Categorical variables included sex, Child class, patient status at transplantation (i.e., in the hospital or in the intensive care unit vs. at home), and status of underlying liver parenchyma (i.e., cirrhotic vs. healthy liver). For donors and grafts, continuous variables included age, body weight, and preharvesting levels of bilirubin, ALAT, ASAT, and gamma glutamyl transpeptidase (GGT) (IU/L). Categorical variables included sex, presence of severe steatosis of the graft, and type of graft (full vs. left vs. right). For comparing the matching of donors to recipients, the donor-to-recipient body weight ratio (%) and the graft-to-recipient body weight ratio (%) were calculated and then classed as less than 1% or 1% or more. 6,27 Other factors potentially associated with outcome were assessed, including cold ischemia time (minutes), units of packed red cells transfused during transplantation, duration of intensive care unit stay, and total hospital stay. Postoperative complications considered to be related to surgical technique, whatever their delay of onset, were classified into the following categories: postoperative hemorrhage requiring reoperation, biliary, arterial, portal, or other.
Outcomes for right and left split-liver grafts were compared with each other and with whole-liver graft outcomes in elective first transplants with whole livers from optimal donors. The main end points were patient survival and graft survival at 1 and 2 years. Variables of potential prognostic importance were assessed by univariate analysis. If significantly correlated with the main end points (log-rank test), they were evaluated in the Cox multivariate logistic regression proportional hazard model, using forward stepwise selection to identify independent predictive factors. Risk ratios (RR) and corresponding 95% confidence intervals (CI) were calculated. P < .05 was considered significant. All statistical analyses were performed using StatView 4.5 software (Abacus Concepts, Inc., Berkeley, CA).
RESULTS
Logistic Feasibility
Thirty-six split-liver grafts were obtained from 18 donors. Of these, 30 were transplanted in our center. Two right and two left split-liver grafts were transplanted at two other centers. Two split-liver grafts (one right and one left) were discarded because of prolonged ischemia, because the first transplant procedure was much longer than planned and a second team was not available.
Donor–Recipient Matching
There were 16 donors, median age 40 (range 23–55) years, all men. One split-liver procedure was performed in a donor with a GGT level of 362 IU/L (reference range for men, 11–85) whose liver had a macroscopic appearance of mild steatosis. Subsequent graft histology showed the steatosis to be massive. The two FAP domino donors, both men, were aged 40 and 52 years and were both at home before transplantation. The cadaver donors for these two patients did not meet the criteria for a split procedure.
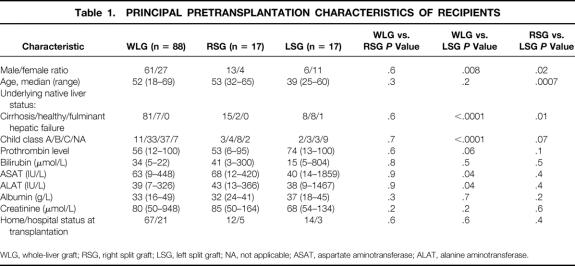
The 34 split-liver grafts were transplanted to 19 male and 15 female recipients, median age 47 (range 25–65) years. Recipients of whole-liver and right split-liver grafts were comparable in all aspects (Table 1). Recipients of left split-liver grafts were younger than recipients of right split-liver grafts (P = .0007). More of them had native livers not affected by cirrhosis than recipients of right split-liver grafts or whole-liver grafts, and their pretransplant liver function as assessed by Child class, ASAT, and ALAT was better, with the exception of the one case of fulminant hepatic failure.
Table 1. PRINCIPAL PRETRANSPLANTATION CHARACTERISTICS OF RECIPIENTS
WLG, whole-liver graft; RSG, right split graft; LSG, left split graft; NA, not applicable; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase.
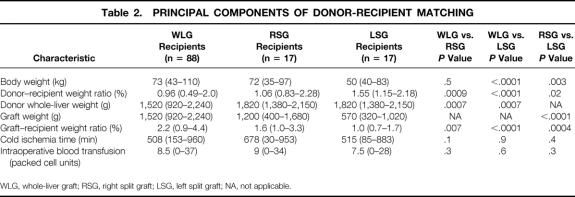
The main aspects of donor–recipient matching are summarized in Table 2. Recipients of whole-liver grafts and right split-liver grafts were comparable in terms of body weight, cold ischemia time, and intraoperative blood transfusion, but recipients of left split-liver grafts were significantly lighter, with a median body weight of 50 kg. Recipients of left split-liver grafts were comparable to recipients of right split-liver grafts and whole-liver grafts in terms of cold ischemia time and blood transfusion, but the grafts were smaller, and the graft-to-recipient body weight ratios were lower than in recipients of right split-liver grafts and whole-liver grafts. The median donor whole-liver weight in the whole-liver group was less than in the split-liver group, with a wider range.
Table 2. PRINCIPAL COMPONENTS OF DONOR-RECIPIENT MATCHING
WLG, whole-liver graft; RSG, right split graft; LSG, left split graft; NA, not applicable.
Early Outcomes
Six patients died in the hospital after SLT (median transplantation interval 2.6 months, range 1.3–7). Death was technique-related in one patient (intraperitoneal bile leak on day 5, persisting despite repeat laparotomies, death at 7 months from sepsis and multiple organ failure). Death was not attributable to the split-liver technique in five patients (causes of death: acute respiratory distress syndrome, multiple organ failure, bacterial pneumonia, herpesvirus hepatitis, multiple organ failure after retransplantation). No inhospital deaths occurred after whole-liver transplantation.
Primary nonfunction occurred in 3 of the 17 left split-liver grafts, in none of the 17 right split-liver grafts, and in none of the 88 whole liver transplants (right vs. left, P = .07; left vs. full, P < .0001). The corresponding figures for graft-to-recipient body weight ratio were 0.87%, 0.86%, and 0.69% (an auxiliary partial orthotopic liver transplantation). One occurred in a steatotic liver. All patients underwent a retransplant within 5 days of transplantation with whole cadaver grafts, but one patient died of septic complications related to the retransplantation.
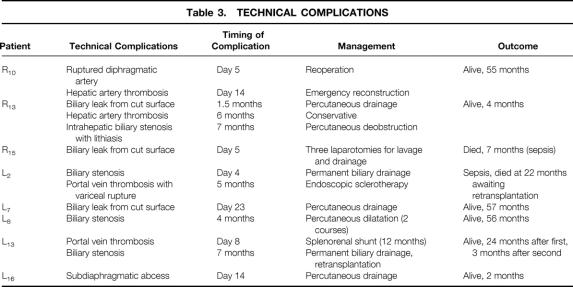
Twenty-six patients (76%) had no technical complications after SLT. Thirteen technical complications occurred in eight patients (five left and three right), leading to an overall complication rate 24% for SLT. Table 3 summarizes the nature, management, and outcomes of the complications, which led to graft loss in one patient and to two patient deaths (at 7 and 22 months after transplantation).
Table 3. TECHNICAL COMPLICATIONS
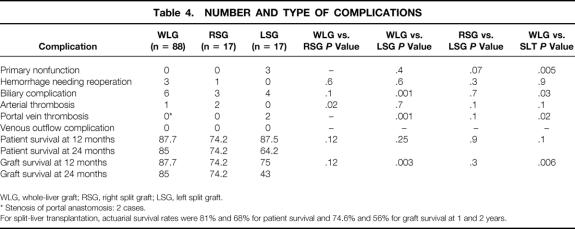
Ten technical complications occurred in 9 of the 88 whole-liver recipients (complication rate, 10%): postoperative hemorrhage (n = 3), biliary complications (n = 6), and an arterial thrombosis (n = 1). Two cases of portal vein stenosis were diagnosed by Doppler ultrasonography and were considered as not hemodynamically significant. The only arterial complication lead to graft loss (intrahepatic cholangitis), with successful retransplantation at 24 months. The number and type of complications seen in split-liver and whole-liver transplantations are compared in Table 4.
Table 4. NUMBER AND TYPE OF COMPLICATIONS
WLG, whole-liver graft; RSG, right split graft; LSG, left split graft.
* Stenosis of portal anastomosis: 2 cases.
For split-liver transplantation, actuarial survival rates were 81% and 68% for patient survival and 74.6% and 56% for graft survival at 1 and 2 years.
Late Outcomes
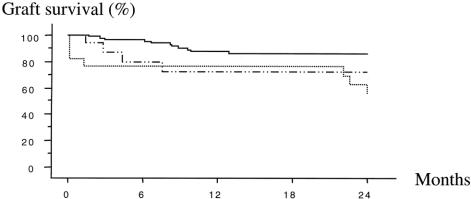
There was no difference in graft survival between whole-liver grafts and right split grafts, but the survival of left split grafts was significantly lower at 2 years (43%) compared with whole-liver grafts (85%;P = .003;Fig. 2).
Figure 2. Actuarial graft survival. Whole liver, uninterrupted line; left split graft, dotted line; right split graft, dashed line.
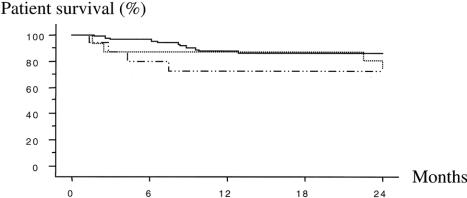
There was no significant difference in inpatient survival rates whether patients received whole-liver grafts or right or left split-liver grafts at 1 year (87.7%, 74.2%, 87.5%) or at 2 years (85.0%, 74.2%, 64.0%;Fig. 3).
Figure 3. Actuarial patient survival. Whole liver, uninterrupted line; left split graft, dotted line; right split graft, dashed line.
Two recipients of a left split-liver graft died within 24 months of SLT, after hospital discharge. The patient who had undergone the flop implantation procedure developed early biliary obstruction that required permanent biliary drainage. A portal vein thrombosis was diagnosed at 5 months. This patient died of sepsis at 22 months while awaiting retransplantation. This death was ascribed to the surgical technique. The second patient developed intrahepatic and portal pedicle lymphoproliferative disease at 5 months after an uneventful SLT. Chemotherapy led to necrosis of an intrahepatic nodule communicating with the bile ducts. The patient needed permanent biliary drainage and had repeated cholangitis. He died of sepsis and multiple organ failure 24 months after SLT.
Factors Associated with Patient and Graft Survival
Univariate analysis showed six factors associated with patient survival: recipient status at transplantation (P = .0001), elevated donor GGT level (P = .0004), graft steatosis (P < .0001), graft-to-recipient body weight ratio (P = .015), intensive care unit stay (P = .0005), and total hospital stay (P < .0001). There was no association with the recipient’s sex, age, liver parenchyma status, Child class, or any of the biochemical parameters in the pretransplantation workup. There was no significant influence of the type of graft, the cold ischemia time, intraoperative blood transfusion, or the ratio of the graft size to the patient’s body weight.
The logistic regression model showed that patient survival was reduced when there was graft steatosis (P < .0001; RR 125, 95% CI 16–1,000), when recipients were in hospital or in the intensive care unit (P = .02; RR 3.6, 95% CI 1.3–10.4), or when the hospital stay was long (P = .007; RR 1.008, 95% CI 1.002–1.014).
Univariate analysis showed 10 factors inversely associated with graft survival: the recipient’s hospital status at transplantation (P = .0001); recipient pretransplant levels of bilirubin (P = .017), ASAT (P = .001), and ALAT (P = .001); donor GGT level (P = .001); steatosis of the graft (P < .0001); a left graft (P = .003); graft-to-recipient body weight ratio less than 1% (P < .0001); duration of intensive care unit stay (P = .0007); and total hospital stay (P < .0001).
Univariate analysis showed no significant association with graft survival of recipient sex, age, status of underlying liver parenchyma, Child class, creatinine, prothrombin time, albumin, donor parameters such as age, bilirubin, ASAT, ALAT, donor-to-recipient body weight ratio, cold ischemia time, or amount of intraoperative transfusion. The logistic regression model showed that graft steatosis (P = .002, RR 25, 95% CI 3–200), graft-to-recipient body weight ratio less than 1% (P = .001, RR 6.5, 95% CI 2.1–20), and total hospital stay (P < .0001, RR 1.01, 95% CI 1.002–1.008) were independently associated with graft failure.
DISCUSSION
Three cases of SLT for two adults reported previously 6 are included in this series because the donors were optimal. Only three case histories have been found in the literature, including one from our center. 13,28,29 The outcomes of these patients are not comparable because of the clinical status of the donors and recipients.
Patient and Graft Survival Rates
For right split-liver grafts, we found patient and graft survival rates comparable to those for whole-liver transplantation. The recipients of a left split graft in this series had a higher risk of graft failure from primary nonfunction. Patient survival rates were comparable, taking into account the three retransplantations necessary because of primary nonfunction.
Donor–Recipient Matching
The fact the left graft recipients were younger, smaller, and had better native liver function simply reflects the surgeons’ selection bias for left split-liver graft recipients. The principal pretransplantation factors influencing patient survival were graft steatosis and the hospital status of the recipient. The two principal factors affecting graft survival were graft steatosis and a graft-to-recipient body weight ratio of less than 1%.
The graft failure resulting from steatosis is not surprising. In retrospect, the donor liver with steatosis should not have been split. We appreciated at the time that the liver was marginal, but the deteriorating clinical condition of the recipient and the shortage of grafts for adults led to the decision to make the split based on macroscopic appearance and texture rather than on histologic examination, which would have added to the ischemia time.
We had previously established that a graft-body weight ratio of less than 1% is associated with a less favorable outcome in SLT. 6,27 This was confirmed in the present analysis by a 6.5-fold (CI 2.1–20) increase in the risk of graft failure when the ratio was less than 1%. Essentially, this means that the potential recipient of a left split-liver graft should have a body weight corresponding to the surgeon’s weight-range estimate of the donor left split graft.
We had expected that the loss of functional parenchyma resulting from cold ischemia 30 would be additive with small graft size as a cause of primary nonfunction or anastomotic failure. However, we avoided transplantation of organs with very long cold ischemia times, so the lack of an observable effect of cold ischemia time in this series is not unexpected. The much shorter cold ischemia time in the in situ split procedures in the FAP patients, however, suggests that cadaver livers should also be split in situ.
The use of small left split grafts as auxiliary transplants in heavier patients is arguable. In adults, the principal indication is fulminant hepatic failure, 24 but the outcomes of these emergency procedures is poor, with graft and patient survival rates in adults of 29% and 35%, respectively. 31 The one auxiliary partial orthotopic liver transplant in this series had early graft failure. Our conservative conclusion is that split livers should not be used for auxiliary partial orthotopic liver transplant procedures, especially in emergency situations such as fulminant hepatic failure.
Recipients had a higher risk of death (RR 3.6, 95% CI 1.3–10.4) if they were in the hospital (either in the intensive care unit or a regular hospital bed) than when at home. An elective transplantation procedure in a patient normally at home appears to be a better indication, with a risk of death 3.6 times less than urgent transplantation in an inpatient. The importance of elective procedure was confirmed by Busuttil (personal communication) in a recent multivariate analysis of his updated series. Previously, there was no distinction between the outcomes of elective and urgent procedures. 3,29
Duration of hospital stay was, logically, related to both patient and graft survival. The relation was small but significant and resulted from the nature and severity of the complications.
Surgical Technique
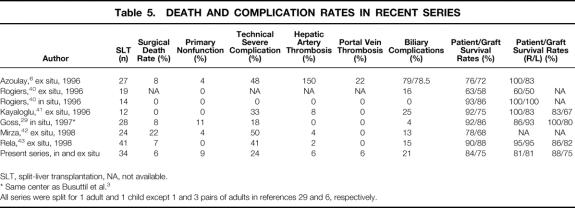
Table 5 summarizes reported complication rates in conventional SLT for an adult and a child.
Table 5. DEATH AND COMPLICATION RATES IN RECENT SERIES
SLT, split-liver transplantation, NA, not available.
* Same center as Busuttil et al. 3
All series were split for 1 adult and 1 child except 1 and 3 pairs of adults in references 29 and 6, respectively.
The reported rates of arterial occlusion in whole-liver transplantation range from 0% to 6% in adults 32–35 and from 3.2% to 16.7% in children (2.8–11.4% for reduced-size liver grafts). 32–34,36–39 In SLT, these range from 0% to 15%. 6,29,40–43 The rate of arterial complications in the present series (6%) is within the range of the present state of the art. The application of microsurgical techniques to the SLT procedure should optimize the results, as reported for living-related liver transplantation. 29 Venous outflow obstruction for left split grafts did not occur using the venoplasty techniques. 22 We have no hypothesis to explain the two cases of portal vein thrombosis in this series.
Biliary complications occurred in 22% of patients in this series. Published rates range from 12.5% to 19.5% in adults 32,44,45 and from 5% to 22% in children (5–13% in reduced-size liver grafts). 33,36,38,39,45 Biliary complications occurred in 0% to 25% of recent series of SLT. 6,29,40–43 There are two types of biliary complications. The more benign are leaks from the raw surface, which usually resolve with percutaneous drainage, although one death resulted from the complications of interventions for a persistent leak. Problems concerning the biliary anastomosis are more troublesome, especially stenosis. The three cases here all occurred in a left split graft, in a choledochojejunostomy, and were relieved by percutaneous balloon dilatation and temporary stenting. The application of microsurgical techniques to the biliary reconstruction could reduce the incidence of biliary complications, but there are other approaches. The dissection of the left hepatic duct and the hilar plate can be minimized to avoid devascularization. Stenting of the anastomosis has been reported in living-related liver transplantation. 46 Constructing the anastomosis on a Roux-en-Y loop on the right side takes advantage of the healthier vascular supply of the intrahepatic right duct. Most importantly for the further expansion of split-liver procedures for two adults is to reduce the cold ischemia time by splitting the liver in situ in the cadaveric donor. Preliminary results with this procedure have shown a low incidence of biliary problems. 3,29,40
Expanded Use
This analysis shows that split-liver procedures for two adults are technically feasible. The recipient of a right split graft is not penalized in terms of graft survival, patient survival, or complications. In two of the three cases of primary nonfunction, a subcritical mass of parenchyma was transplanted, and the third had a steatotic liver. These events may be avoided in future left split graft recipients by rigid selection criteria.
Taking into account retransplantations, 89 whole-liver grafts were transplanted into 88 recipients, and 21 livers (18 split, 3 whole) were transplanted into 34 patients. In this series, SLT yielded a 62% net increase in adult transplant recipients. The potential gain in the number of patients transplanted is actually even greater because two split grafts could not be used for logistic reasons, and at least two of the split-liver failures requiring retransplantation could have been avoided by rigid selection criteria.
More widespread use of split-liver procedures for two adults would also improve outcomes through experience. The outcomes in publications since 1995 3,6,29,40–43 are better than those in earlier reports, 15,21,39,47–49 and it is logical to expect the same in splits for two adults. There is also a clear volume-to-outcome relation in liver transplantation, 50 which would also be expected with SLT. In the European Liver Transplant Registry, there is a clear relation between the volume of SLT procedures performed and the outcome: 1-year patient and graft survivals are significantly better in centers performing more than 40 SLT procedures (77% and 67% respectively) than in centers performing 20 to 40 SLT procedures (67% and 58%. 51 Additional arguments in favor of establishing split-liver programs in large centers are related to logistics and potential recipients. In this series, a split procedure was performed in only 16 of 106 (15%) eligible cadaver donors. In our center, the second transplant team is usually assembled from off-duty volunteers, which is not always possible. Greater familiarity with SLT in neighboring centers could increase the numbers of split grafts sent out when a local second team is unavailable.
A second limitation is the availability of size-matched recipients. The criteria for matching the recipient to the graft tend to select lighter recipients, with better underlying liver function. The potential recipients of a left split-liver graft of partner centers could be pooled.
Sex matching may be necessary for wider practice of SLT for adults. The optimal donor is more likely to be male because of the selection by body weight (100% of donors in this series). Similarly, low-body-weight recipients of left split grafts are more likely to be female (65% of left graft recipients in this series) and younger. Male-donor-to-female-recipient liver transplantation has a higher association with chronic rejection, particularly in recipients with primary biliary cirrhosis, 52 which is an indication also likely to be found in the split graft recipient population. Male-donor-to-female-recipient liver transplantation also appears to have a higher incidence of posttransplant malignancies. 53 One female patient died of complications of lymphoproliferative disease within 24 months of a split-liver transplant from a male donor, but there is no clear evidence of a role of sex mismatch in this outcome.
We conclude that the liver should definitely be considered as often as possible as a paired organ. SLT for two adults is feasible and can provide outcomes equivalent to those of whole-liver transplantation. There are no medical or ethical obstacles to more widespread use. Changes are needed in the way that organs are allocated and in the coordination between transplant centers to maximize the potential for reducing the graft shortage for adults.
Acknowledgment
The authors thank the transplant coordinating nurses from the teams at Beaujon Hospital (Clichy) and Mondor Hospital (Créteil) for assistance with follow-up data from split-liver recipients.
Footnotes
Correspondence: Dr. Daniel M. Azoulay, Centre Hépato-Biliaire, Hôpital Paul Brousse, 94804, Villejuif, France.
E-mail: daniel.azoulay@pbr.ap-hop-paris.fr
Accepted for publication October 2, 2000.
References
- 1.Smith CME. Annual report of the U.S. Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network: transplant data, 1988–1996. UNOS, Richmond, VA, and the Division of Transplantation, Bureau of Health Resources and Services Administration, U.S. Department of Health and Human Services. Rockville, MD: 1997.
- 2.Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet 1999; 354: 1636–1639. [DOI] [PubMed] [Google Scholar]
- 3.Busuttil RW, Goss JA. Split liver transplantation: a review. Ann Surg 1999; 229: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transplant 2000; 6: 3–20. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay D, Bismuth H. Living donor liver transplantation. Present and future alternatives [in French]. Gastroentérol Clin Biol 2000; 24: 782–790. [PubMed] [Google Scholar]
- 6.Azoulay D, Astarcioglu I, Bismuth H, et al. The split liver transplantation: the Paul Brousse policy. Ann Surg 1996; 224: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azoulay D, Samuel D, Castaing D, et al. Domino liver transplants for metabolic disorders: experience with familial amyloidotic polyneuropathy. J Am Coll Surg 1999; 189: 584–593. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay D, Castaing D, Adam R, et al. Transplantation of three adult patients with one cadaveric graft: wait or innovate. Liver Transplant Surg 2000; 6: 239–240. [DOI] [PubMed] [Google Scholar]
- 9.Makuuchi M, Kawasaki S, Nogushi T, et al. Donor hepatectomy for living related partial liver transplantation. Surgery 1993; 113: 395–402. [PubMed] [Google Scholar]
- 10.Yamaoka Y, Washida M, Honda K, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994; 57: 1127–1130. [PubMed] [Google Scholar]
- 11.Colledan M, Andomo E, Valente U, et al. A new splitting technique for liver grafts [letter]. Lancet 1999; 353: 1763. [DOI] [PubMed] [Google Scholar]
- 12.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation einer Spenderleber auf zwei Empfänger (Splitting-Transplantation). Eine neue Methode in der Weiterentwicklung der Lebersegmenttransplantation. Langenbecks Arch Chir 1988; 373: 127–130. [PubMed] [Google Scholar]
- 13.Bismuth H, Morino M, Castaing D, et al. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg 1989; 76: 722–724. [DOI] [PubMed] [Google Scholar]
- 14.Otte JB, de Ville de Goyet J, Alberti D, et al. The concept and technique of the split liver in clinical transplantation. Surgery 1990; 107: 605–612. [PubMed] [Google Scholar]
- 15.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver. Analysis of a preliminary experience with “split liver” grafting. Ann Surg 1990; 21: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki CF, Langnas AN, Bynon JS, et al. A percutaneous method for venovenous bypass in liver transplantation. Transplantation 1994; 57: 472–473. [PubMed] [Google Scholar]
- 17.Calne RY, William R. Transplantation in man. I. Observations on technique and organization in five cases. Br Med J 1968; 4: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lerut J, de Ville de Goyet J, Donataccio M, et al. Piggyback transplantation with side-to-side cavocavostomy is an ideal technique for right split liver allograft implantation. J Am Coll Surg 1994; 179: 573–576. [PubMed] [Google Scholar]
- 19.Shaw BW Jr, Iwatsuki S, Starzl TE. Alternative methods of hepatic graft arterialization. Surg Gynecol Obstet 1984; 159: 490–493. [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw BW Jr, Iwatsuki S, Bron K, et al. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet 1985; 161: 67–68. [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw BW, Wood RP, Stratta RJ, et al. Management of arterial anomalies encountered in split-liver transplantation. Transplant Proc 1990; 22: 420–422. [PubMed] [Google Scholar]
- 22.Emond JC, Heffon TG, Whitington PF, et al. Reconstruction of the hepatic vein in reduced-size hepatic transplantation. Surg Gynecol Obstet 1993; 176: 11–17. [PubMed] [Google Scholar]
- 23.Dunn SP, Langham MR Jr, Marmon LM. A new approach to the left lateral segment hepatic transplant: the flop. Transplantation 1990; 49: 660–662. [DOI] [PubMed] [Google Scholar]
- 24.Bismuth H, Azoulay D, Samuel D, et al. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann Surg 1996; 224: 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living donor liver transplantation: its surgical advantages compared to conventional procedures. Transplantation 1992; 54: 263–268. [DOI] [PubMed] [Google Scholar]
- 26.Inomoto T, Nishizawa F, Sasaki H, et al. Experience with 120 microsurgical recontructions of hepatic artery in living related liver transplantation. Surgery 1996; 119: 20–26. [DOI] [PubMed] [Google Scholar]
- 27.Adam R, Castaing D, Bismuth H. Transplantation of small donor livers in adult recipients. Transplant Proc 1993; 25: 1105–1106. [PubMed] [Google Scholar]
- 28.Moreno-Gonzalez E, Gomez SR, Garcia GI, et al. Utilization of split-liver grafts in orthotopic liver transplantation. Hepato-Gastroenterol 1993; 40: 17–21. [PubMed] [Google Scholar]
- 29.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation 1997; 64: 871–877. [DOI] [PubMed] [Google Scholar]
- 30.Adam R, Bismuth H, Diamond T, et al. Effect of extented ischemia with UW solution on graft function after liver transplantation. Lancet 1992; 340: 1373–1376. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus P, Bechtein WO. Split liver/auxiliary liver transplantation for fulminant hepatic failure. Liver Transplant Surg 1997; 3 (suppl 1): S55–61. [PubMed] [Google Scholar]
- 32.Szapakowski JL, Cox K, Nakazato P, et al. Liver transplantation: experience with 100 cases. West J Med 1991; 155: 494–501. [PMC free article] [PubMed] [Google Scholar]
- 33.Busuttil RW, Seu PH, Millis JM, et al. Liver transplantation in children. Ann Surg 1991; 213: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langnas AN, Marujo W, Stratta RJ, et al. Vascular complications after orthotopic liver transplantation. Am J Surg 1991; 161: 76–83. [DOI] [PubMed] [Google Scholar]
- 35.Mor E, Schwartz ME, Sheiner PA, et al. Prolonged preservation in University of Wisconsin solution associated with hepatic artery thrombosis after orthotopic liver transplantation. Transplantation 1993; 56: 1399–1413. [DOI] [PubMed] [Google Scholar]
- 36.Houssin D, Soubrane O, Boillot O, et al. Orthotopic liver transplantation with a reduced-size graft: an ideal compromise in pediatrics ? Surgery 1992; 111: 532–542. [PubMed] [Google Scholar]
- 37.Lomas DJ, Britton PD, Farman P, et al. Duplex Doppler ultrasound for the detection of vascular occlusion following liver transplantation in children. Clin Radiol 1992; 46: 38–42. [DOI] [PubMed] [Google Scholar]
- 38.Valayer J, Gauthier F, Yandza T, et al. Chirurgie de la transplantation hépatique chez l’enfant. Pédiatrie 1993; 48: 139–141. [PubMed] [Google Scholar]
- 39.De Ville de Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation 1993; 5: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 40.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg 1996; 224: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayaloglu M, D’Alessandro AM, Knechtle SJ, et al. Preliminary experience with split liver transplantation. J Am Coll Surg 1996; 182: 381–387. [PubMed] [Google Scholar]
- 42.Mirza DF, Achilleos O, Pirenne J, et al. Encouraging results of split-liver transplantation. Br J Surg 1998; 85: 494–497. [DOI] [PubMed] [Google Scholar]
- 43.Rela M, Vougas V, Muiesan P, et al. Split liver transplantation. King’s College Hospital experience. Ann Surg 1998; 227: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stratta RJ, Wood RP, Langnas AN, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery 1989; 106: 675–684. [PubMed] [Google Scholar]
- 45.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994; 219: 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K, Uemoto S, Tokunaga Y, et al. Surgical techniques and innovations in living related liver transplantation. Ann Surg 1993; 217: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houssin D, Boillot O, Soubrane O, et al. Controlled liver splitting for transplantation in two recipients: technique, results and perspectives. Br J Surg 1993; 80: 75–80. [DOI] [PubMed] [Google Scholar]
- 48.Sloof MJH. Reduced-size liver transplantation, split liver transplantation, and living related liver transplantation in relation to the donor organ shortage. Transplant Int 1995; 8: 65–68. [DOI] [PubMed] [Google Scholar]
- 49.De Ville de Goyet J. Split liver transplantation in Europe, 1988 to 1993. Transplantation 1995; 59: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 50.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med 1999; 341: 2049–2053. [DOI] [PubMed] [Google Scholar]
- 51.European Transplant Registry. Data analysis 05/1968–06/1999. Villejuif: ELTR; 1999.
- 52.Candinas D, Gunson BK, Nightingale P, et al. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet 1995; 346: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 53.Jonas S, Neumann U, Guckelberger O, et al. Donor-recipient sex matching and posttransplant malignancies after induction therapy in hepatic transplantation. Transplant Proc 1998; 30: 1490–1491. [DOI] [PubMed] [Google Scholar]