Abstract
Objective
To evaluate the impact of graft size on recipients in living donor liver transplantation (LDLT) to establish a clinical guideline for the minimum requirement.
Summary Background Data
Although the minimum graft size required for LDLT has been reported to be 30% to 40% of graft volume (GV)/standard liver volume (SLV), the safety limit of the graft size was unknown.
Methods
A total of 33 cases of LDLT, excluding auxiliary transplantation, were reviewed with a minimum observation period of 4 months. The 33 patients were divided into three groups according to GV/SLV: medium-size graft group, small-size graft group, and extra-small graft group. The effect of GV/SLV on graft function, graft regeneration, and survival was evaluated.
Results
The overall patient survival rate was 94% at a mean follow-up of 15 months with a minimum observation period of 4 months. There were no statistically significant differences in postoperative bilirubin clearance, alanine aminotransferase, prothrombin time, and frequency of postoperative complications among the three groups. One week after transplantation, the regeneration rate (GV at 1 week/harvested GV) in the extra-small and small groups was significantly higher than that of the medium group. The graft and patient survival rates were both 100% in the extra-small group, 75% and 88% in the small group, and 90% and 95% in the medium group.
Conclusions
Small-for-size grafts less than 30% of SLV can be used with careful intraoperative and postoperative management until the grafts regenerate.
Since the first success in 1989, 1 living donor liver transplantation (LDLT) has been refined and accepted as a valuable treatment for patients with terminal liver disease. 2 Although LDLT was originally developed as a response to the shortage of pediatric donors, this procedure has recently been extended to adult patients in countries where the availability of cadaveric donors is severely restricted. 3 Accompanying these trends, there has been a move toward increasing the size of harvested grafts, from an extended lateral segment to an extended left lobe, and to right lobe grafts. 4 However, donor safety in LDLT must remain a prerequisite. The major concern in using LDLT for adult patients is the adequacy of the size of the graft that can be safely harvested from the donor. The Shinshu University group accepted a donor–recipient combination that gives an estimated graft volume (GV)/standard liver volume (SLV) ratio of more than 30%. 3 Lo et al 5 reported that a graft with GV/SLV of 40% or less should be regarded as a marginal graft that would have a lower success rate. However, experience with adult-to-adult LDLT is limited, and the minimum graft size remains unknown. In the current study, we investigated whether a small GV/SLV ratio was correlated with a poorer outcome in LDLT.
PATIENTS AND METHODS
Patient Population
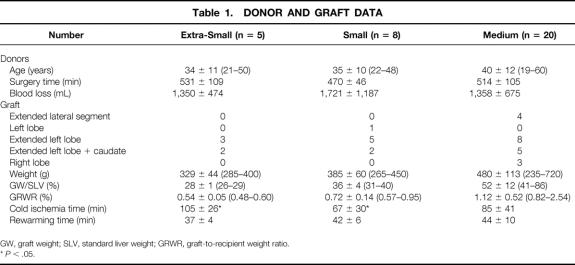
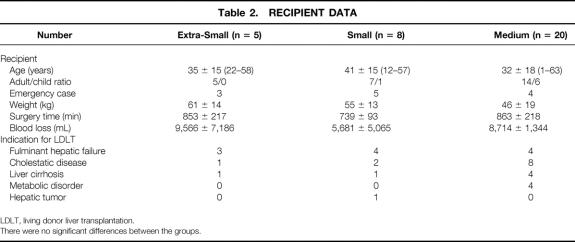
From October 1996 to January 2000, LDLT was performed on 35 patients at the Kyushu University Hospital with the approval of the Kyushu University Ethics Committee. Among them, auxiliary transplantations (n = 2) were excluded, and the remaining 33 cases with a minimum observation period of 4 months were included in the analysis. The patients comprised 14 male patients and 19 female patients, ranging in age from 1 to 63 years (mean ± SD, 35 ± 17; median 40). Their body weight ranged from 10 to 84 kg (51 ± 18, median 52). Data on the donors and recipients, classified by graft volume as defined later, are shown in Tables 1 and 2.
Table 1. DONOR AND GRAFT DATA
GW, graft weight; SLV, standard liver weight; GRWR, graft-to-recipient weight ratio.
* P < .05.
Table 2. RECIPIENT DATA
LDLT, living donor liver transplantation.
There were no significant differences between the groups.
Evaluation of Graft Size
Standard liver volume was calculated according to the formula by Urata et al. 6 The weight of the harvested liver segment, assigned as the GV, was measured on the back table, and GV/SLV was also calculated. 7 Computed tomography (CT) performed 1 week after transplantation was used for GV analysis. The regeneration rate at 1 week after transplantation and the GV at 1 week/SLV (%) were determined from the CT.
Choice of Graft for Hepatectomy
For a child patient, an extended lateral segment or left lobe of the donor is chosen as a graft. For an adult patient, if the donor’s extended left lobe, estimated by preoperative CT, is more than 30% of GV/SLV, it is chosen. If the predicted GV/SLV is approximately 30%, the left side of the caudate lobe is included in an attempt to increase the functional volume. If the donor’s predicted extended left lobe with the caudate lobe is less than 30% and approximately 25% of GV/SLV, a right lobe graft or auxiliary partial graft is chosen.
Groups
The 33 patients were divided into three groups according to their GV/SLV ratios: medium-size graft group, GV/SLV more than 40% (group M, n = 20), small graft group, GV/SLV 30% to 40% (group S, n = 8), and extra-small graft group, GV/SLV 30% or less (group ES, n = 5).
Surgical Technique
Donor hepatectomy and recipient transplantation procedures were performed as described elsewhere 2,3 with minor modifications. The volume of the donor liver segment was calculated before surgery by CT. The choice of resected segments for donation was dictated by the need to obtain a graft volume of more than approximately 30% of the recipient’s standard liver volume, and included the extended left lateral segment, left lobe, extended left lobe, extended left lobe with left caudate lobe, and right lobe. In the donor procedure, parenchymal dissection was performed with or without intermittent inflow occlusion of the glissonian pedicle in the remnant side of the hepatic lobe. After parenchymal transection, the liver graft was flushed in situ and preserved in cold University of Wisconsin solution. In the recipient procedure, the native liver was resected, preserving the inferior vena cava. A temporary portofemoral bypass or portocaval shunt was made during the anhepatic phase in noncirrhotic patients with circulatory instability, as indicated by a clamping test on the portal vein or the recipient having a GV/SLV of less than 30%. The portocaval shunt was established using anastomosis of the right branch of the portal vein to the inferior vena cava in end-to-side fashion, while the portal flow was maintained through the left branch of the portal vein. The portocaval shunt was taken down after reperfusion of the graft through the left branch of the portal vein. Consequently, the flow of the portal vein was not interrupted through the surgical procedure.
After reconstructing the hepatic vein and portal vein, the hepatic artery was anastomosed under surgical microscopy. The biliary tract was reconstructed by hepaticojejunostomy using a Roux-en-Y loop.
Postoperative Care
The initial immunosuppressive regimen consisted of tacrolimus and steroids. Coagulation was controlled intensively for the first week; low-dose heparin and fresh-frozen plasma were administered and the prothrombin time and activated clotting time were monitored. Fresh-frozen plasma was given during and after LDLT in patients with a prolonged prothrombin time of 20 seconds or more.
Serum levels of total bilirubin and alanine aminotransferase (ALT) and prothrombin time were evaluated before surgery and on days 1, 3, 5, and 7 after transplantation. Posttransplant complications including intractable ascites (requiring drainage >1 month after LDLT), intraabdominal bleeding, vascular complications, biliary complications, acute rejection, and relaparotomy were evaluated. Graft and patient survival were also evaluated. The graft regeneration rate (posttransplant 1 week GV [mL]/harvested GV [mL]) was determined.
Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed using the Student t test. Differences at P < .05 were considered significant.
RESULTS
Donors and Recipients
The mean GW/SLV was 52 ± 12% in group M, 36 ± 4% in group S, and 28 ± 1% in group ES. The lowest GW/SLV was 26% and the highest 86%. Surgical time and blood loss during donor and recipient surgery did not differ between the groups. Cold ischemia time was 85 ± 41 minutes in group M, 67 ± 30 minutes in group S, and 105 ± 26 minutes in group ES. Cold ischemia time in group S was significantly shorter than that in group ES (P < .05).
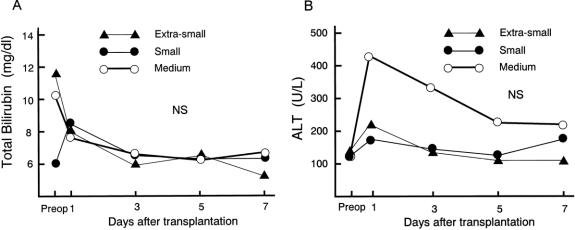
Posttransplant Graft Function
The postoperative serum level of total bilirubin was similar and the postoperative serum level of ALT was the same for the three groups (Fig. 1). The prothrombin time was prolonged on day 1 after transplantation in all groups. There were no statistically significant differences between the postoperative prothrombin times of the groups. One patient with a 26% GV/SLV received one session of plasma exchange on day 1 after LDLT: the prothrombin time was 27.4 seconds just before plasma exchange and decreased to less than 20 seconds on day 2 after LDLT. There were no significant differences in postoperative complications between the groups (Table 3). Graft and patient survival also did not differ significantly.
Figure 1. Posttransplant graft function parameters: total bilirubin, alanine aminotransferase, and prothrombin time. There were no significant differences in these values between the groups.
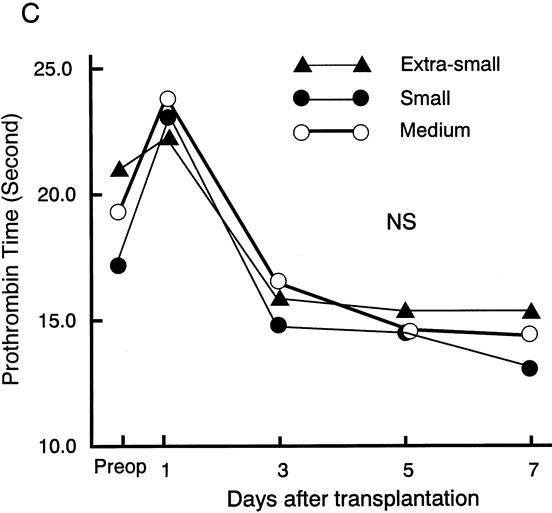
Figure 1. Continued
Table 3. COMPLICATIONS AND SURVIVAL
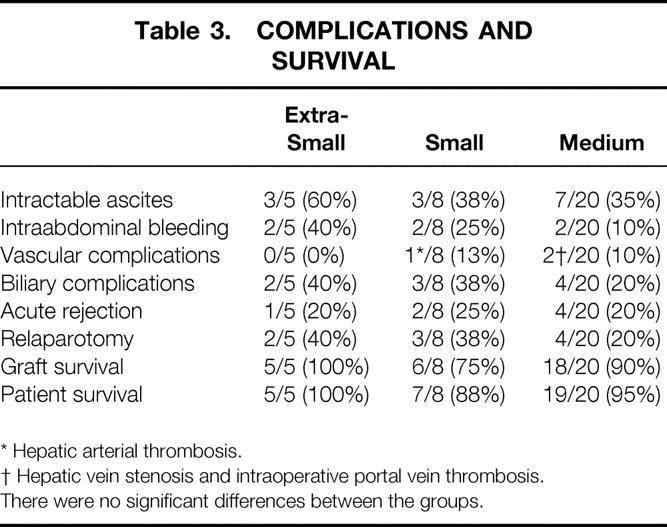
* Hepatic arterial thrombosis.
† Hepatic vein stenosis and intraoperative portal vein thrombosis.
There were no significant differences between the groups.
In group ES, two patients required relaparotomy, one for anastomotic bleeding at the jejunojejunostomy of a Roux-en-Y loop and the other for decreased portal vein flow. The latter subsequently underwent splenectomy to reduce portasystemic shunt vessels. In group S, three patients had relaparotomy, two for intraabdominal bleeding and the other for hepatic artery thrombosis. In group M, four patients had relaparotomy, two for bile leakage, one for intraabdominal bleeding, and the other for hepatic vein stenosis.
In group ES, there was no graft loss, and all five patients did well. There were two graft losses in group S. One graft loss was due to the death of the recipient from adult T-cell leukemia 6 months after LDLT. The other recipient developed hepatic artery thrombosis and underwent a second LDLT 1 month after the first. In group M, two grafts were lost. One was due to the death of the recipient, who developed sudden-onset hepatic infarction 4 months after LDLT. The other patient developed Budd-Chiari syndrome as a result of stenosis of the hepatic venous anastomosis and underwent a second LDLT 11 months after the first.
Graft Liver Regeneration
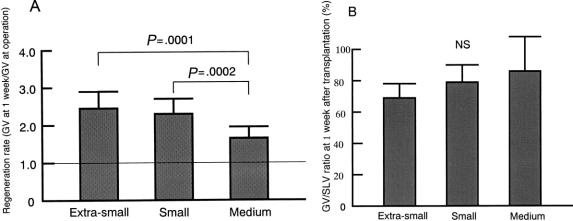
The regeneration rate of the graft, defined as the GV at 1 week after surgery/harvested GV, is shown in Figure 2. One week after transplantation, the regeneration rates in groups ES and S were significantly greater than in group M (P = .0001 and .0002, respectively). There were no significant differences among the three groups in the GV/SLV at 1 week after LDLT.
Figure 2. (A) Regeneration rate, graft volume (GV) at 1 week/GV at surgery. Regeneration rates in the extra-small and the small groups were significantly higher than those in the medium-size group. (B) GV/standard liver volume (SLV) 1 week after transplantation. There were no significant differences between the groups.
DISCUSSION
One of the most important points to consider in LDLT is the minimum GV needed to meet metabolic demand. According to experience gained in hepatic surgery, an extended hepatectomy of up to 80% to 85% of the whole liver can be tolerated in noncirrhotic patients. 8 This means that 15% to 20% of the liver can support hepatic function. However, this cannot be translated directly into the lower limit of graft volume in LDLT, which requires periods of cold and warm ischemia and subsequent reperfusion of the graft. Shirakata et al 9 reported that the minimum limitation of the graft size for successful liver transplantation was approximately 25% on the basis of the orthotopic partial liver transplantation in a canine model. The reported minimum GV for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure is 25% of GV/SLV. 10 Kawasaki et al 3 reported that a ratio of 30% for GV/SLV can maintain good viability and regeneration after hepatectomy in clinical cases. Recently, Lo et al 5 reported that a graft with 40% or less of GV/SLV should be regarded as a marginal graft, with a lower success rate. Kiuch et al 11 reported that graft size less than 1% of recipient body weight leads to lower graft survival. However, with limited data on adult-to-adult LDLT, the minimum GV remains unknown.
In adult-to-adult LDLT, the choice of the donor hepatectomy is a key element in the success of LDLT. If the donor’s extended left lobe estimated by preoperative CT is more than 30% of GV/SLV in an adult recipient, we use an extended left lobe graft. If the predicted extended left lobe of the donor is approximately 30% of SLV, we add the left side of the caudate lobe in an attempt to increase the functional volume. However, if the donor’s predicted extended left lobe with the caudate lobe is much less than 30% and approximately 25% of GV/SLV, a right lobe graft or auxiliary partial graft would be considered. The use of a right lobe graft may provide a greater functional hepatic volume. However, a donor with a predicted GV/SLV of less than 30% usually has a proportionally large right lobe compared with the left lobe, which might mean that the right lobe consists of 70% or more of the donor whole liver. Harvesting a right lobe in such a donor may increase the risk of death and complications. An auxiliary partial graft may be another choice. An auxiliary liver in a patient with, for example, fulminant hepatic failure, cannot be expected to work as a support of hepatic function, because the recipient’s hepatic function is almost zero. In some instances, the harvested GV might be smaller than predicted because of calculation error from the CT scan. The difference between predicted and harvested GV might be attributable to respiratory movement of the diaphragm, the heartbeat, and use of a cutting line different from that defined before surgery. In group ES, GV/SLV was 29% in two patients (liver cirrhosis and fulminant hepatic failure), 27% in two patients (primary sclerosing cholangitis and fulminant hepatic failure), and 26% in one patient (fulminant hepatic failure). In all five patients in group ES, predicted GV was 30% or more of SLV. The recipient’s total hepatectomy is performed under temporary portosystemic shunt with preservation of congestion of the intestine when the actual GV/SLV is less than 30%. Ku et al 12 reported an improved survival of canine liver transplantation using a quarter-graft with the aid of a portohepatic vein shunt. The effect of a portohepatic vein shunt on portal vein decompression should be an important factor for preventing graft injury after recirculation in an extremely small graft. 12 One hundred five minutes of cold ischemia time was needed in group ES because of construction of the portosystemic shunt.
Our results showed that all recipients who received grafts with a GV/SLV ratio less than 30% survived with good postoperative hepatic function. The postoperative serum levels of total bilirubin and ALT showed no significant difference among the three groups. This means that the small-for-size graft could sustain injury after the portocaval shunt was taken down. Although two grafts were lost in group S, no grafts were lost because of hepatic dysfunction related to the small graft volume.
The GV/SLV ratio increased from 27% to 67% in 1 week after LDLT in group ES. The regeneration rate was significantly greater compared with group M. No significant difference was found in postoperative prothrombin time among the three groups. However, fresh-frozen plasma was given during and after LDLT to support hepatic protein synthesis in patients with prolonged prothrombin time of 20 seconds or more. One patient with a 26% GV/SLV received one session of plasma exchange on day 1 after LDLT, and the prothrombin time decreased to less than 20 seconds on day 2 after LDLT. Yamanaka et al 13 reported that synthesis of albumin recovery was delayed compared with the recovery of the liver volume and bilirubin clearance after hepatectomy. They speculated that protein synthesis is used to reconstruct the liver parenchyma rather than to excrete albumin after large hepatic resection.
In conclusion, our data show that a small graft with a GV/SLV ratio of 29% to 26% can be used with support for hepatic synthetic function by administering fresh-frozen plasma and using a portosystemic shunt for portal vein decompression.
Footnotes
Supported in part by a grant-in-aid for general research from the Ministry of Education, Japan.
Correspondence: Takashi Nishizaki, MD, Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812-8582, Japan.
E-mail: nishi@surg2.med.kyushu-u.ac.jp
Accepted for publication October 3, 2000.
References
- 1.Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990; 322: 1505–1507. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka K, Uemoto S, Tokunaga Y, et al. Surgical techniques and innovations in living related liver transplantation. Ann Surg 1993; 217: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg 1998; 227: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg 2000; 231: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation 1999; 68: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 6.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995; 21: 1317–1321. [PubMed] [Google Scholar]
- 7.Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Validation and a comparison of the two techniques. Gastroenterology 1985; 88: 1812–1817. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Putnam CW, Groth CG, et al. Alopecia, ascites, and incomplete regeneration after 85 to 90 percent liver resection. Am J Surg 1975; 129: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirakata Y, Terajima H, Mashima S, et al. The minimum graft size for successful orthotopic partial liver transplantation in the canine model. Transplant Proc 1995; 27: 545–546. [PubMed] [Google Scholar]
- 10.Lo CM, Fan ST, Chan JK, et al. Minimum graft volume for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure. Transplantation 1996; 62: 696–698. [DOI] [PubMed] [Google Scholar]
- 11.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999; 67: 321–327. [DOI] [PubMed] [Google Scholar]
- 12.Ku Y, Fukumoto T, Nishida T, et al. Evidence that portal vein decompression improves survival of canine quarter orthotopic liver transplantation. Transplantation 1995; 10: 1388–1392. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993; 18: 79–85. [PubMed] [Google Scholar]