Abstract
Objective
To determine whether parenteral feeding (IV-TPN) influences the local and systemic response to an intestinal insult.
Summary Background Data
Parenteral feeding increases ICAM-1 expression and attracts neutrophils (PMNs) to the intestine compared with enterally fed animals. Because the gut is a priming bed for PMNs, the authors hypothesized that IV-TPN may affect organ injury after gut ischemia—reperfusion (I/R).
Methods
Mice were randomized to chow, IV-TPN, intragastric TPN, or complex enteral diet for 5 days’ feeding. In experiment 1, 162 mice underwent 15 or 30 minutes of gut I/R, and death was recorded at 72 hours. In experiment 2, 43 mice underwent 15 minutes of gut ischemia and permeability was measured by 125I-labeled albumin at 3 hours after reperfusion. Lung PMN accumulation was measured by myeloperoxidase assay. In experiment 3, albumin leak was tested in the complex enteral diet group (n = 5) and the intragastric TPN group (n = 5) after 30 minutes of gut ischemia and 1 hour of reperfusion.
Results
In experiment 1, enteral feeding significantly reduced the death rate compared with IV-TPN after 15 minutes of I/R. After 30 minutes of gut ischemia, the IV-TPN and intragastric TPN groups showed a higher death rate than the chow and enteral diet groups. In experiment 2, IV-TPN significantly increased pulmonary and hepatic 125I albumin leak compared with enteral feeding without increasing pulmonary myeloperoxidase levels. In experiment 3, there were no differences in 125I albumin leak between the complex enteral diet and intragastric TPN groups.
Conclusion
Enteral feeding reduced the death rate and organ permeability after 15 minutes of ischemia. However, prolonged ischemia (30 minutes) eliminated any benefits of intragastric TPN on survival.
Enteral delivery of nutrients benefits severely injured or critically ill patients more than parenteral nutrition (IV-TPN) by reducing infectious complications in the respiratory tract and intraabdominal cavity. 1–3 This advantage has been shown experimentally using models of bacterial or viral challenge to the respiratory tract 4,5 or bacterial instillation into the peritoneal cavity. 6 Mice immunized with antigens against a specific virus or bacteria maintain respiratory immunity against the virus or bacteria when fed enterally, whereas parenteral feeding impairs this protection. 4,5 Rats fed intragastric TPN (IG-TPN) survive intraperitoneal injection of Escherichia coli by inhibiting bacterial proliferation in the peritoneal cavity and reducing bacteremia compared with rats receiving IV-TPN. 6
Our recent data show that IV-TPN affects the vascular endothelium (as well as the mucosal surfaces) by upregulating ICAM-1 and P-selectin expression, which causes an interaction between polymorphonuclear neutrophils (PMNs) and endothelium 7,8 and increases intestinal PMN accumulation. Extraintestinal organs, such as the lung and kidney, also show higher adhesion molecule expression with IV-TPN than with enteral feeding. We speculated that the vascular changes induced by IV-TPN might affect the response to an injury such as ischemia/reperfusion (I/R). Gut ischemic insults frequently occur in clinical settings as a result of disproportionate splanchnic hypoperfusion during shock. 9–11 It is believed that this phenomenon is a critical initiating event for multiple organ dysfunction after severe traumatic injuries. 9–11 The intestinal vascular bed serves as a priming bed of circulating PMNs. Primed PMNs accumulate in remote organs, particularly in the lung, and injure the tissues if a second insult occurs. 9,12
Therefore, the purpose of this study was to examine the effects of type and route of nutrition on the host response to gut I/R using four diets that affect mucosal and vascular endothelial integrity: chow, a complex enteral diet (CED), IG-TPN (as a model of an elemental diet), and IV-TPN. Survival rate, survival time, and vascular permeability in remote organs were chosen as indicators of host resistance to the gut I/R insult.
MATERIALS AND METHODS
Animals
All experimental protocols were approved by the Animal Care and Use Committee of The University of Tennessee. Male ICR (Institute of Cancer Research) mice were purchased from Harlan (Indianapolis, IN) and housed in a conventional facility accredited by the American Association for Accreditation of Laboratory Animal Care. The environment was controlled with regard to temperature and humidity, with a 12-hour light/dark cycle. Mice were given free access to chow (RMH3200, Agway, Syracuse, NY) and water for 2 weeks before entry into this study protocol. During feeding protocols, mice were housed in metal metabolism cages with wire-grid floors to eliminate coprophagia.
Feeding Protocol
Experiment 1
This experiment was designed to assess the effects of the route and type of nutrition on the death rate after gut ischemic insults. One hundred sixty-two mice (6–8 weeks old) were randomized before cannulation to receive chow (n = 30), IV-TPN (n = 54), IG-TPN (n = 49), or CED (n = 29). Because pilot studies showed a high survival rate in CED or chow groups after 15 minutes of I/R, fewer animals were randomized to those groups. In mice randomized to the chow and IV-TPN groups, internal jugular catheters were inserted under anesthesia with ketamine hydrochloride (100 mg/kg) and acepromazine maleate (10 mg/kg). Through a right jugular approach, a silicone rubber catheter (0.3 mm ID, 0.6 mm OD; Baxter, Chicago, IL) was inserted into the vena cava. The proximal end of the catheter was tunneled subcutaneously over the spine and exited the tail at its midpoint. The mice were placed into metal metabolism cages and partially immobilized by tail restraint to protect the catheter during infusion. The technique does not induce physical or biochemical stress. 13 Mice randomized to IG-TPN or CED received gastrostomy tubes. Through a vertical midline incision, the stomach was delivered into the wound. A 7-0 silk pursestring suture was placed on the posterior wall of the stomach, followed by a gastrotomy using a 25-gauge needle. The silicone catheter (0.5 mm ID, 0.9 mm OD; Baxter) was inserted into the gastrotomy. Omentum was mobilized and free-tied around the insertion of the gastrostomy tube using 7-0 silk suture. The proximal end of the catheter was tunneled subcutaneously over the spine and exited the tail at its midpoint.
Catheterized mice were immediately connected to infusion pumps (Instech Laboratories, Plymouth Meeting, PA), and received 0.9% saline at 4 mL/day for 48 hours with free access to chow and water. On postoperative day 2, mice received their respective feeds. Chow-fed animals received 4 mL 0.9% saline IV along with free access to chow and water throughout the study. The IV-TPN and IG-TPN animals initially received 4 mL/day TPN and were advanced to a goal rate of 11 mL/day by the third day of feeding. The TPN solution contained 4.1% amino acids, 34.3% glucose (4,878 kJ/L), electrolytes, and multivitamins, with a nonprotein calorie:nitrogen ratio of 743 kJ/g nitrogen. This feeding met the calculated nutritional requirement of mice used in the present study. TPN mice received 2,146 kJ/kg nonprotein calories per day and 18.0 g protein/kg per day. The CED mice received 6 mL/day Isocal (Mead Johnson Co., Evansville, IN), with an increase in rate to a goal of 13 mL/day. Isocal contains 13.3% carbohydrate, 4.5% fat, and 3.4% protein (4,441 KJ/L), in addition to electrolytes and vitamins. The nonprotein calorie:nitrogen ratio of Isocal is 709 kJ/g nitrogen. Thus, the administered diets were almost isocaloric and isonitrogenous, except for the chow mice.
After receiving their respective diets for 5 days, animals were anesthetized with ketamine hydrochloride and acepromazine maleate given subcutaneously. A 2.5-cm-long midline laparotomy was performed and the superior mesenteric artery (SMA) was identified. The SMA was occluded with a microvascular clip for 15 (n = 106) or 30 minutes (n = 56). The laparotomy incision was immediately closed during SMA occlusion and reopened to remove the clip. Survival was observed until 72 hours after reperfusion (Fig. 1). All mice were resuscitated with a subcutaneous injection of 1 mL saline solution before reperfusion and had free access to chow and water during the observation of survival.
Figure 1. Protocol for survival study. CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition; IV-TPN, intravenous total parenteral nutrition; SMA, superior mesenteric artery; s.c., subcutaneous.
Experiment 2
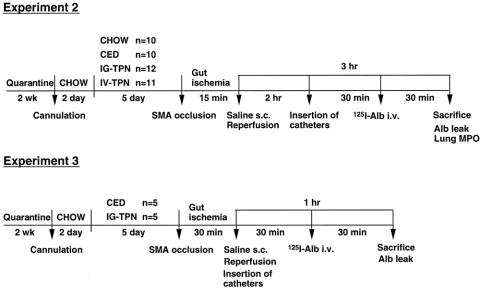
This experiment was designed to assess the effects of route and type of nutrition on distant organ injury and pulmonary myeloperoxidase activity after 15 minutes of gut I/R (Fig. 2). After 5 days of diet (chow n = 10, CED n = 10, IG-TPN n = 12, IV-TPN n = 11), the mice underwent 15 minutes of SMA occlusion, 1 mL saline resuscitation, and 3 hours reperfusion. Distant organ vascular permeability was measured with 125I-labeled bovine serum albumin. PMN accumulation in the lungs was assessed by myeloperoxidase activity measurement in the lungs.
Figure 2. Protocol for vascular permeability study. CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition; IV-TPN, intravenous total parenteral nutrition; SMA, superior mesenteric artery; s.c., subcutaneous; Alb, albumin; i.v., intravenous; MPO, myeloperoxidase.
Experiment 3
This experiment was designed to assess the effects of type of nutrition on distant organ injury after gut I/R. The CED (n = 5) and IG-TPN (n = 5) mice were studied 1 hour after 30 minutes of SMA occlusion, and 1-mL saline resuscitation, to examine distant organ injury with 125I-labeled bovine serum albumin. One hour was chosen because of the high death rate.
Vascular Permeability in Organs
The left jugular vein and the right carotid artery were cannulated with a silicone rubber catheter and polyethylene tubing (PE10, Becton Dickinson, Sparks, MD). Twelve micrograms 125I-labeled albumin (3 million to 5 million cpm) in a volume of 200 μL phosphate-buffered saline was given through the jugular vein catheter (total volume, 200 μL). A blood sample was obtained through the carotid artery catheter 30 minutes after injection of the albumin. Then the animals were heparinized (40 U sodium heparin) and rapidly exsanguinated by perfusion of bicarbonate-buffered saline through the jugular vein catheter with simultaneous blood withdrawal through the carotid artery catheter. This was followed by perfusion of 15 mL bicarbonate-buffered saline through the carotid artery catheter after severing the inferior vena cava at the thoracic level to remove all 125I-labeled albumin that had not leaked into the interstitium. The lungs, liver, kidney, heart, pancreas, and stomach were harvested, washed in water, and blotted dry.
Calculation of Permeability Index
The Cobra Automated Gamma Counting System (Packard Instrument, Meriden, CT) was used to count 125I-labeled albumin activity in each tissue and in a 50-μL blood sample. After measuring radioactivities, all tissues were placed in the oven for 3 days and weighed. Results are expressed as a permeability index in the equation: Permeability index = (cpm/g dry tissue)/(cpm/g blood).
Measurement of Myeloperoxidase Activity
Tissue myeloperoxidase activity was determined in the left lung. After perfusion with bicarbonate-buffered solution, the left lung was harvested, rinsed, blotted dry, weighed, and frozen at −80°C. The samples were thawed and homogenized in 10 volumes of 0.45% saline solution. The homogenate was centrifuged at 20,000 g for 15 minutes at 4°C. The supernatant was then discarded and the pellet was homogenized with 10 volumes of phosphate-buffered solution (pH = 6.0) containing 0.5% hexadecyl trimethyl ammonium bromide (Sigma, St. Louis, MO). Samples were freeze-thawed (for 20 minutes at −80°C) and sonicated, followed by centrifugation at 20,000 g for 15 minutes at 4°C. The supernatant myeloperoxidase activity was assayed by measuring the H2O2-dependent oxidation of 3,3',5,5'-tetramethylbenzidine. The absorbance was measured at 655 nm (Perkin-Elmer 50B, Oakbrook, IL). Tissue myeloperoxidase activity was expressed as activity units per gram wet weight.
Statistics
The log-rank test was used for survival time comparisons. The Fisher exact probability test was used for survival rate comparisons at 72 hours after reperfusion. Permeability index and myeloperoxidase levels are expressed as means ± standard error and were analyzed using analysis of variance, followed by the Fisher protected least significant difference post hoc test or the Student t test.
RESULTS
Experiment 1
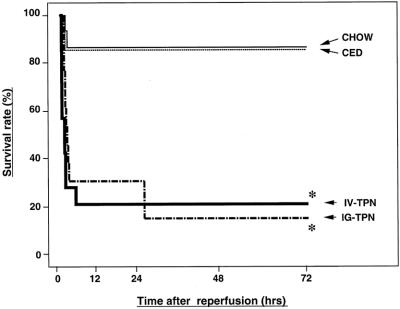
After 15 minutes of SMA occlusion, survival times in the enterally fed groups (chow, CED, and IG-TPN) were significantly improved compared with the IV-TPN group (Fig. 3). The survival rates at 72 hours after reperfusion were 93% (14/15) for the chow and CED groups, 89% (32/36) for the IG-TPN group, and 60% (24/40) in the IV-TPN group (P < .02 vs. chow and CED, P < .004 vs. IG-TPN) (Fig. 4).
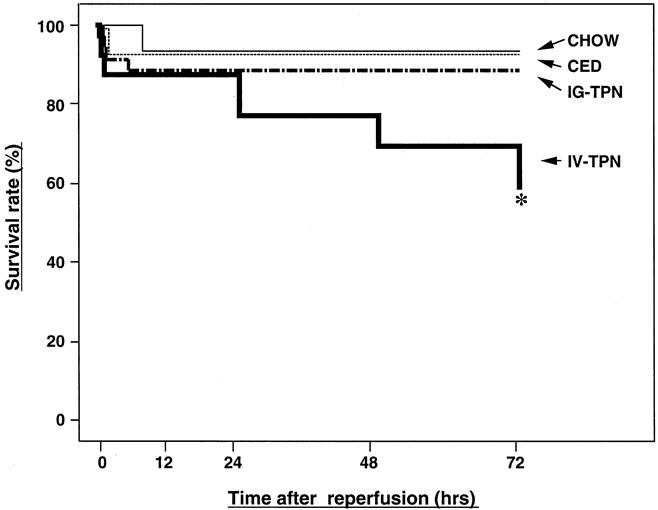
Figure 3. Survival curves for the chow, complex enteral diet (CED), intragastric total parenteral nutrition (IG-TPN), and intravenous total parenteral nutrition (IV-TPN) groups after 15 minutes of superior mesenteric artery occlusion. Survival of the IV-TPN group was significantly less than all enterally fed groups (* P < .03 versus chow and CED, P < .01 versus IG-TPN).
Figure 4. Survival curves for the chow, complex enteral diet (CED), intragastric total parenteral nutrition (IG-TPN), and intravenous total parenteral nutrition (IV-TPN) groups after 30 minutes of superior mesenteric artery occlusion. Survival of the IV-TPN and IG-TPN groups was significantly less than the chow and CED groups (* P < .001 versus chow and CED).
The 30 minutes of SMA occlusion did not decrease survival times of the chow and CED groups compared with 15 minutes of ischemia. However, the survival time of the IG-TPN group was reduced to the level of the IV-TPN group. The survival rates at 72 hours were 87% (13/15), 86% (12/14), 15% (2/13), and 21% (3/14) for the chow, CED, IG-TPN, and IV-TPN groups, respectively (P < .001 IG-TPN or IV-TPN vs. chow and CED).
Experiment 2
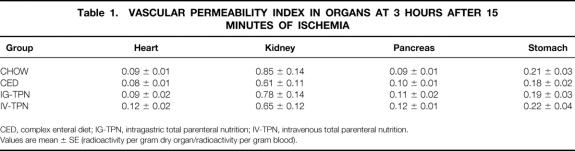
The vascular permeability index in the lung (Fig. 5) and liver (Fig. 6) was significantly higher in the IV-TPN group than in all enterally fed groups. There were no significant differences in the index of other tissues (heart, kidney, pancreas, or stomach) among all four diet groups (Table 1). The wet weight/dry weight ratio was consistently higher in the IV-TPN mice but failed to reach statistical significance (data not shown). There were no significant differences in the myeloperoxidase activity of the lung harvested at 3 hours after 15 minutes of ischemia between any groups (chow, 135.1 ± 17.6; CED, 181.1 ± 16.3; IG-TPN, 166.1 ± 26.7; IV-TPN, 159.3 ± 17.8).
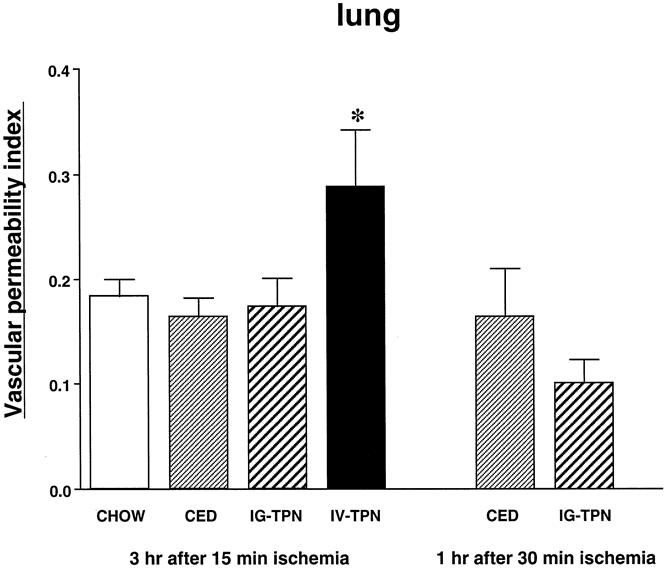
Figure 5. Vascular permeability index in the lung at 3 hours after 15 minutes of superior mesenteric artery occlusion and at 1 hour after 30 minutes of superior mesenteric artery occlusion. CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition; IV-TPN, intravenous total parenteral nutrition. * P < .05 versus chow, CED, and IG-TPN.
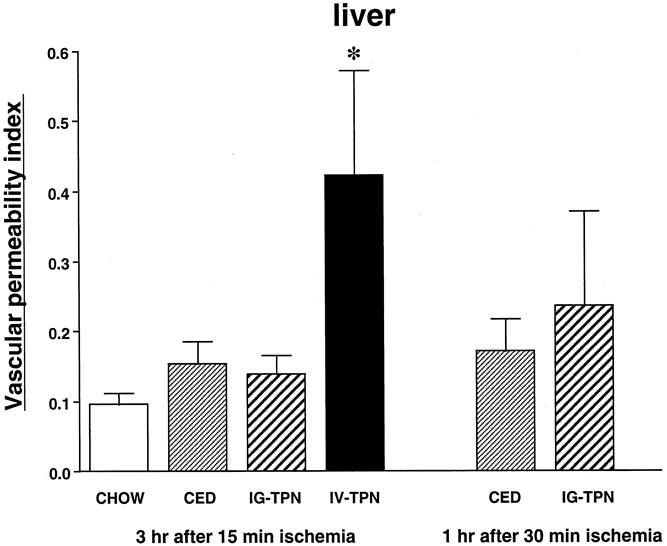
Figure 6. Vascular permeability index in the liver at 3 hours after 15 minutes of superior mesenteric artery occlusion and at 1 hour after 30 minutes of superior mesenteric artery occlusion. CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition; IV-TPN, intravenous total parenteral nutrition. * P < .05 versus chow, CED, and IG-TPN.
Table 1. VASCULAR PERMEABILITY INDEX IN ORGANS AT 3 HOURS AFTER 15 MINUTES OF ISCHEMIA
CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition; IV-TPN, intravenous total parenteral nutrition.
Values are mean ± SE (radioactivity per gram dry organ/radioactivity per gram blood).
Experiment 3
No significant differences were observed in the vascular permeability index of any organs between IG-TPN and CED groups (Table 2).
Table 2. VASCULAR PERMEABILITY INDEX IN ORGANS AT 1 HOUR AFTER 30 MINUTES OF ISCHEMIA
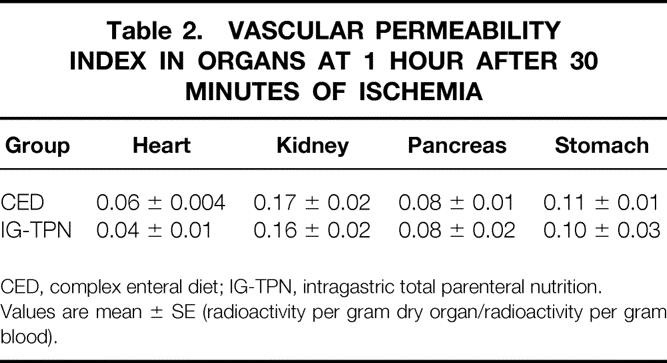
CED, complex enteral diet; IG-TPN, intragastric total parenteral nutrition.
Values are mean ± SE (radioactivity per gram dry organ/radioactivity per gram blood).
DISCUSSION
Clinically, lack of enteral delivery of nutrients results in a higher rate of infectious complications. 2,3,14 Bacterial translocation, microaspiration of pathogens from the gastrointestinal tract, impaired pulmonary macrophage function, and increased gut permeability have been postulated to be responsible for this susceptibility during IV-TPN feeding. 15–19 Our laboratory has proposed a cogent mechanism for the reduced pneumonia rate in enterally fed patients compared with parenterally fed patients: namely, preservation of IgA-mediated mucosal immunity. 4,5,20
Mucosal immunity, which constitutes approximately 50% of total body immunity, is maintained through complex interactions between T cells, B cells, cytokines, and adhesin molecules. In our experiments, these interactions were found to change after dietary manipulations altering the route, type, and complexity of diets. 20 Within 24 hours of lack of enteral feeding and IV-TPN, cell numbers in the gut-associated lymphoid tissue drop, reaching a nadir after 3 to 4 days of parenteral feeding. 21 In this time frame, levels of IgA-stimulating cytokines (interleukin [IL]-4 and IL-10) drop in the Peyer’s patches and lamina propria 22 as intestinal and respiratory 5 IgA levels drop and established antiviral 4 and antibacterial respiratory immunity becomes impaired. These changes reflect significant depression in generalized mucosal immunity. IV-TPN allows the physiologic study of dietary changes on this system, which would be impossible in fasted mice over this time period. A 2-day fast results in 15% weight loss and lethargy; a 3-day fast is lethal. Fasted animals are too weak to sustain any significant metabolic or immunologic challenge. Mice receiving IV-TPN maintain weight and metabolic vigor as changes in complex mechanisms of mucosal immunity occur without the confounding effects of malnutrition. It is unlikely that the TPN solution itself induces toxic effects on mucosal immunity because exogenous administration of bombesin, a neuropeptide normally released by the gastrointestinal tract in response to feeding, completely reverses the deleterious effect of IV-TPN on mucosal immunity. 23
Although enteral nutrition maintains mucosal immunity and resistance to infections, effects on distant organ function in response to nonseptic insults have not been studied, and to the best of our knowledge this is the first demonstration that enteral feeding prevents remote organ injury and death after a gut ischemic insult. Gut I/R secondary to splanchnic hypoperfusion is a common event after a variety of clinical conditions such as trauma, shock, cardiac, or aortic surgery. 9,10 After an initial insult, circulating PMNs are primed in the intestinal vascular bed by various inflammatory mediators, including complement, leukotriene B4, and platelet activating factor. 24–27 Primed neutrophils are then activated if a second insult ensues to attack vital organs and cause multiple organ failure. Moore et al 9 examined the kinetics of lung vascular permeability after gut I/R in a rat model. Forty-five minutes of ischemia increased lung injury evaluated by 125I albumin leakage at 2 and 6 hours after reperfusion, but not at 0 or 1 hour. Based on this report, in a preliminary study, we occluded the SMA for 45 minutes and allowed a 3-hour reperfusion to measure albumin leakage in organs. Chow-fed mice survived this insult, but it was highly lethal to IV-TPN and IG-TPN mice, necessitating a lesser degree of insult in our mouse models. Fifteen minutes of ischemia resulted in a high rate of survival in all animals fed via the gastrointestinal tract but a significantly reduced survival rate in animals receiving IV-TPN. This 15-minute ischemic event resulted in significant increases in lung and liver permeability after 3 hours of reperfusion only in the IV-TPN mice, with no vascular permeability changes in other organs.
Surprisingly, there was no obvious PMN accumulation as measured by myeloperoxidase activity in the lung to account for this permeability increase, suggesting other mediators. Several possibilities exist. Our previous work shows that IV-TPN induces endothelial changes by upregulating ICAM-1 and P-selectin expression in the intestine and E-selectin expression in the lung. 7,8 These changes are associated with PMN accumulation in the intestine but not the lung. The results of the current work suggest that lack of enteral feeding increases the endothelial sensitivity of lung and liver endothelium to other proinflammatory stimulants. However, regardless of total lung myeloperoxidase activity, it is also possible that PMNs that circulated in the blood or accumulated in the tissue were activated more in the IV-TPN group than in the other enteral groups. 28
In addition to PMNs, lipid mediators, phospholipase A2 (PLA2), and various cytokines, such as IL-1, IL-6, IL-8, tumor necrosis factor (TNF), platelet activating factor, and interferon-gamma (IFNγ), have been implicated as a source for tissue injury after release from hypoperfused splanchnic tissue, either through direct effects on the organs or indirectly through PMN activation. 24–27 PLA2 plays a role in many cellular processes, including membrane remodeling, free fatty acid production, eicosanoid generation, and signal transduction. It factors as an important component in the pathophysiology of acute respiratory distress syndrome by inducing a lung injury that can be inhibited by anti-PLA2 therapy. 29 IL-1, TNFα, and IFNγ act synergistically to enhance inducible nitric oxide synthetase and nitric oxide production, which increases cultured enterocyte monolayers and presumably intestinal permeability. 30 TNFα is produced by the Kupffer cells of the liver after I/R and increases leukocyte adhesion. 31 Hydrogen peroxide produced by postanoxic endothelial cells activates T cells, causing the release of TNFα. 32 Gut I/R also causes TNFα release from pulmonary macrophages and the release of IL-6 from the gut. 33,34 IL-8, IFNγ, and leukotriene B4 (a byproduct of PLA2 eicosanoid production) also prime and activate free oxygen radical production in PMNs. Platelet activating factor, produced by the Kupffer cells in the liver, mediates leukocyte-induced hepatocyte injury and increases the production of IL-1 and IL-6 after gut I/R. Inhibitors of platelet activating factor attenuate this response. 31 Thus, many of the products produced by the gastrointestinal tract may be influenced by the cellular changes associated with the gut-associated lymphoid tissue.
It has been postulated that these substances are carried by the thoracic duct lymph, because the acute lung insult to splanchnic hypoperfusion is ameliorated when mesenteric lymph is prevented from reaching the lung. 35 Our observation that the liver is simultaneously affected without changes in vascular permeability of other tissues, such as the kidney, pancreas, and stomach, would suggest that the portal vein also delivers these proinflammatory substrates. One would expect more diffuse distant organ permeability changes if the liver were injured by only inflammatory mediators that were carried only by the thoracic duct but not cleared by the lung and allowed to reach the systemic circulation. Of course, there is also the potential that these organs are not susceptible to these inflammatory agents.
Interestingly, 30 minutes of ischemia eliminated any beneficial effect of IG-TPN on survival, suggesting that whatever protection was present with this “elemental” diet at 15 minutes was lost after 30 minutes of ischemic insult but was still adequate in animals receiving chow or CED. A host of possibilities warrant further investigation of differences between the gastrointestinal tracts of mice receiving IG-TPN given 15-minute and 30-minute insults, including the availability of cellular antioxidants, the levels of nitric oxide, and the levels of PLA2, in addition to others. Although vascular permeability remained unchanged 1 hour after this 30-minute ischemia, it is possible that the lung does not respond with permeability increases that rapidly. 9 Unfortunately, many animals do not survive 3 hours after this more severe insult to test for more delayed vascular permeability changes. Nonetheless, it does seem clear that increases in vascular permeability are not the cause of death after this more severe insult.
Our understanding of the impact of nutrition support on outcome in critically ill and severely injured patients has progressed significantly during the past two decades. Experimentally, the route of nutrition influences previously established mucosal immunity, gut-associated lymphoid tissue cell populations, intestinal cytokine levels, and intestinal and respiratory IgA levels. The results of the current work suggest that the route of nutrition also influences the inflammatory response to an intestinal ischemic insult after reperfusion. In severely injured patients, complex formulas enriched in ω-3 fatty acids, arginine, or glutamine appear to provide additional benefits, resulting in a reduced incidence of multiple organ dysfunction after injury. 36,37 Each of these substrates exerts specific effects on immunologic and metabolic responses to injury such that a wide variety of mechanisms could potentially be affected. The precise definition of mechanisms influenced by I/R that can be manipulated by enteral nutrition provides an extensive array of processes to be investigated experimentally.
Discussion
DR. JON M. BURCH (Denver, Colorado): It is a pleasure to have the opportunity to discuss this paper by Drs. Kudsk and colleagues, which purports to further the evidence in support of enteric as opposed to parenteral feedings in critically ill patients. Although I am not a basic surgical scientist, I have a strong clinical bias in favor of early enteral nutrition, and my discussion of this paper is akin to choir preaching.
In the first paragraph of the discussion of the manuscript, the authors make the bold statement that this series of experiments is the first evidence that enteral feedings may reduce the risk of multiple organ failure and related mortality in animals at risk. If true, and I have no reason to suspect otherwise, this is a very important observation.
Previous experiments in both animals and humans have demonstrated a decrease in infectious complications but not MOF with the use of enteral feedings. Although we acknowledge a relationship between infection and MOF, to assume that an intervention that inhibits one will necessarily prevent the other is unwarranted. Therein lies the importance of these experiments, although the supporting evidence is somewhat tenuous. One of the early sentinel events in the genesis of MOF is increased vascular permeability and the accumulation of primed neutrophils in the target organs. In the current experiments, enteral feedings inhibited increases in vascular permeability in both the lung and liver. However, neutrophil activity remained unaltered.
My criticism of the authors’ work has to do with the design of the study, which in my view as a clinician, has plagued many similar ventures. In this study, the mice were fed the experimental diets for 5 days before the ischemia/reperfusion insult occurred. Afterward, they were all fed chow prior to sacrifice. In the clinical arena our critically ill patients are only susceptible to dietary interventions after the insult has occurred, not before. There are many examples where preinsult treatment interventions have been entirely effective in the laboratory but marginally so in post-insult interventions in human trial.
Therefore, my question for the authors is why did you select preinsult treatment rather than postinsult treatment in the current series of experiments?
The manuscript is otherwise concise and well written and I commend it to the membership. Finally, I would like to thank the Association and the authors for the privilege of discussing this paper.
DR. WILLIAM G. CIOFFI, JR. (Providence, Rhode Island):
Dr. Kudsk and his colleagues have added to our understanding of the importance of using the gut as the preferred nutritional conduit whenever possible. They have shown today that both the route and type of nutrition is important in this murine model of intestinal ischemia/reperfusion. Enteral nutrition decreased mortality and vascular permeability in the liver and lung, which did not appear to be secondary to a decrease in PMN driven distant organ injury. Towards this end, I have several questions:
First, it would appear that IV-TPN is detrimental when animals are preconditioned with it. In this ‘pretreatment‘ model, is it really the fact that enteral nutrition improves outcome or is it that nutrition really does not matter as long as it is not administered parenterally? Dr. Kudsk attempted to answer this question in his introductory remarks by stating that these animals couldn’t be fasted and studied, but yet we fast our patients routinely for 1, 2, 3, and 4 days before we operate on them – maybe not ideal – and we don’t see this same kind of effect. Do you have data in a larger animal model that would substantiate your claim that it is indeed the enteral route that is important?
Second, what kind of mice did you use? Is this a strain or species specific finding? Do you have other data on organ function or wet to dry weights to corroborate your albumin data? What did these animals die from?
Third, if we accept the conclusion that the provision of enteral nutrition does present some of the distant organ dysfunction which accompanies gut ischemia reperfusion, what are the potential mediators, as your MPO data would indicate that it does not appear to be secondary to effect on PMN priming, which has been shown to accompany this insult.
Data from Deitch and his colleagues suggest that the mediators are in the gut-derived lymph channels and that these are responsible for lung dysfunction, and that division of these lymph channels prevents lung injuries. If this is true, how do you explain your hepatic data?
Finally, animals receive their various nutritional formulas for 5 days prior to gut ischemia reperfusion. Trauma patients do not usually come packaged this way. Is there really a clinical correlate to your model? Patients who develop postoperative sepsis and possible mesenteric ischemia during that septic insult likewise are not really equivalent to your model.
I enjoyed this paper very much, as it certainly fits with my bias concerning nutritional provision, and yet I have several reservations concerning your conclusions.
I thank the Association for the privilege of discussing this paper.
DR. R. NEAL GARRISON (Louisville, Kentucky): I congratulate Dr. Kudsk and colleagues for another clear report on the benefits of enteral feeding.
I am curious, however, as to the local mechanism of this phenomenon. How are nutrients exposed first to the intestinal mucosa better than those exposed to the vascular endothelial cell as a first step in the process, especially when you are showing that the endothelial cell apparently is one of the cells that benefits from this phenomenon.
Is it a direct topical effect or is there a signal pathway to the immunologic and vascular endothelial cell from the enterocyte?
Thank you for the privilege of the floor.
DR. BASIL A. PRUITT, JR. (San Antonio, Texas): I rise to compliment the authors. I also rise to express an alternative hypothesis. It seems entirely consistent with the findings that the nutrients given parenterally are simply toxic and have nothing to do with the route of delivery. It is just a concentration effect, and there is greater concentration when it is given IV than when it is given enterally, and all the findings can be explained by that mechanism.
Thank you.
DR. EDWARD M. COPELAND, III (Gainesville, Florida): I just have a simple thing to ask Ken. First of all, I wonder if he has looked at the effect of gastrin on the gut in any of these models that he has told us about over the years. We showed sometime ago that gastrin prevents the atrophy of the small bowel mucosa when given with TPN. I think our secretary has actually studied bombesin in some of the same research areas and has noted similar effects.
The real question I have for Ken is, over the years, you have told us how bad TPN is. I mean, every year we have a paper on TPN as a harmful therapeutic modality. I want to ask you, do you use TPN in your practice, and if so, under what circumstances?
Is there anything good, Ken, that TPN does?
Thank you.
DR. JOSEF E. FISCHER (Cincinnati, Ohio): Mr. President, Secretary Townsend. It has been a while since I have played around with blood flow studies using labeled materials. But my recollection of my days with blood flow studies using simple labels and the assumptions that we make in this is that it is a very tricky business.
We have a finding here of which little more radiolabeled albumin seemingly ends up outside the vascular space in two organs in which the capillary arrangements are rather complex as compared, for example, to the heart and some of the other organs in which it does not.
I wonder whether or not this particular technique has really been vented in the sense that the washout that you are depending on to really get the albumin out of the vascular space is really adequate in the lung and the liver, which really have fairly complex interstices where things can hide.
The second question I have is – and it is really a pet peeve of mine – is that we have really gotten lost about this multiple organ failure syndrome in the gut. Translocation is a normal process, sort of like a patrol. When you’re on enemy lines, you send out a patrol, you capture a soldier, you interrogate him. What the gut is doing is sending out patrols into the gut saying, okay, here is a bacteria which is likely to invade me. I’m going to grab him, bring him back to a lymph node so I can prepare my defense in case something breaks down.
I think it is unfortunate that we have followed in surgery a pathway by saying gut permeability may be increased, and it probably is increased in burns. Wes Alexander has shown that very nicely. It may be increased in shock, Rusher’s paper, although nobody has identified what the radio activity that Dr. Rusher’s laboratory counted. But to immediately assume that we go from increased capillary permeability in a nonterminal event to multiple organ failure, I think that is a real mistake. There have been 28 studies that I know of for gut sterilization that does not change anything in outcome as far as multiple organ failure, other than pneumonitis from aspiration. So I think we have to be very careful.
And the third thing is that gut feeding is useful in another way, and it increases hepatic protein synthesis, which is every bit important for acute phase reactants and all sorts of other things. We don’t need to get that much of the caloric requirement into the gut. I think 20% to 30%, from various studies carried out, is adequate.
I think this paper is interesting, I think it is important because it challenges convention, and I think those kinds of approaches are always very useful.
Thank you.
DR. KENNETH A. KUDSK (Memphis, Tennessee): Thank you very much. Those are very interesting questions, great questions, and things that I have given a lot of thought to myself.
One of the first questions that got addressed by a number of individuals is, Is IV-TPN bad? Or is there something about enteral feeding that’s good? And although Dr. Copeland said that I have been saying that TPN is bad, I don’t think I have been. I think what I have been saying is that there are benefits that are gained when you deliver nutrients via the gastrointestinal tract that are not gained when you deliver them intravenously. That’s my message.
Is it the nutrients themselves? I think this is a side effect of being fed via the gastrointestinal tract, because, if you give these animals parenteral nutrition and look at any of our functional models—the antibacterial pneumonia, antibacterial defenses, the antiviral defenses, GALT atrophy—and you give those animals bombesin, everything comes back to normal. IV-TPN was designed to get nutrients into the body. It was not designed to maintain the gut associated lymphoid tissue or keep the enteric nervous system stimulated. It very effectively gets the nutrients into the body. But enteral nutrition appears to also stimulate the enteric nervous system, and release the hormones. You can get similar responses with gastrin, you can get similar responses with cholecystokinin, but you can’t get any responses with neurotensin.
One of the problems at the University of Tennessee is we don’t have very many patients on TPN. There are a few on the medical service, a few on the SICU service, but in the trauma center there’s really very few, and they are the desperately sick patients. I think it is an intriguing concept to give neuropeptides to patients who are on parenteral nutrition to see if we can gain that same benefit in mucosal immunity, but I don’t have the patients to do it, because most of my patients are fed enterally, few of them are fed parenterally. People have to fail enteral feeding before they are put on parenteral nutrition, unless we have an absolute disaster that it’s clear GI tract feeding is not going to work.
Dr. Cioffi asked if we have used a larger animal model. The mucosal immune system, in rats, is totally different from humans. The mouse is similar to man. We have used outbred mice, but inbred mice seem to do the same thing. There is a lot of mucosal immunology, basic science literature, to compare our data, and we knew we had things pretty well defined, in terms of cell numbers within the Peyer’s patches and lamina propria, because we obtained the same data as they get in inbred mice. We are starting to do some experiments with F1 mice now in an inbred model.
What is the cause of death? I’m not really sure, but there are some mechanisms that I think are occurring. For example, when you feed animals intravenously, glutathione levels drop. We have an experiment in which we have supplemented IV-TPN with glutamine, and we have improved response and improved survival to the ischemia reperfusion. So glutathione could be affected.
Secondly, there are lipid changes that occur, and we can manipulate these eventually by feeding various lipids via the gastro-intestinal tract to look at different thromboxanes and leukotrienes to see if they are influenced. But we have not done that work yet.
I suspect that this is a function of neutrophils. We do have a paper now which is about to be submitted in which, with IV-TPN, when we do immunohistochemical staining, the animals that are fed intravenously have significantly increased expression of CD18. So although I showed that there is no change in the absolute number of neutrophils within the lung, it does appear that the PNMs in IV-TPN animals are upregulated prior to the insult.
Is there a clinical correlate? Well, I think there’s a lot of patients who come in with an injury that aren’t fed via the gastrointestinal tract prior to a subsequent insult. There are a lot of general surgical patients who are sitting on the wards who are being fed intravenously for 3, 4, 5, 6, 7 days prior to going to surgery, and I think they are clinical correlates.
We are approaching this whole area from a physiologic standpoint to see if we can understand how pertubations induced by not feeding via the gastrointestinal track affects responses and affects the basic aspect of mucosal immunity.
The questions were asked about insults before nutrition support. It becomes a problem with the animal care and use committee. We had to jump through hoops to get this double surgery with cannulation and then ischemia reperfusion. To put another insult, then a cannulation and then another insult on top of that, I don’t know what it would take. It becomes a practical problem. But there may be things we can do with lipo-polysaccharide or some other insults, but we have not induced an insult prior to TPN yet.
In terms of the local mechanisms, I have talked a little bit about the enteric nervous system. It is not a signal from the enterocyte; I believe it is a signal from the enteric nervous system. And I don’t think that it is a toxicity of IV-TPN, Dr. Pruitt, because if you give the neuropeptides, everything returns to normal. So that certainly would go against that hypothesis.
Dr. Fischer, the issues with albumin and blood flow are a tricky business. I think bacterial translocation is an interesting phenomenon. If I want to upregulate the response of the mucosal immune system, all I need to do is give a little cholera toxin with any of the antigens, and it will upregulate that response. I could make a strong argument that bacterial translocation is the body’s attempt to get antigen into the body to upregulate this response similar to the way colera toxin does. I have got data in patients showing that there are increases in permeability associated with increases in IL6, but I don’t think bacterial translocation is a player.
I hope I have answered all the questions, and, again, I appreciate the opportunity to present our data here.
Thank you.
Footnotes
Supported by NIH grant # 5 R01 GM53439.
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Kenneth A. Kudsk, MD, 956 Court Ave., Suite E228, Memphis, TN 38163.
E-mail: kkudsk@utmem.edu
Accepted for publication December 2000.
References
- 1.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med 1991; 325: 525–532. [DOI] [PubMed] [Google Scholar]
- 2.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 1991; 215: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Feliciano DV, Androssy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg 1992; 216: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 1996; 223: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg 1999; 229: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin MT, Saito H, Fukushima R, et al. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg 1996; 223: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukatsu K, Lundberg AH, Hanna MK, et al. Route of nutrition influences ICAM-1 expression and neutrophil accumulation in intestine. Arch Surg 1999; 134: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu K, Lundberg AH, Hanna MK, et al. Increased expression of intestinal P-selectin and pulmonary E-selectin during IV-TPN. Arch Surg 2000; 135: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 9.Moore EE, Moore FA, Franciose RJ, et al. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma 1994; 37: 881–887. [DOI] [PubMed] [Google Scholar]
- 10.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 1999; 178: 449–453. [DOI] [PubMed] [Google Scholar]
- 11.Simpson R, Alon R, Kobzik L, et al. Neutrophil and nonneutrophil mediated-injury in intestinal ischemia–reperfusion. Ann Surg 1993; 218: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koike K, Moore FA, Moore EE, et al. Endotoxin after gut ischemia/reperfusion causes irreversible lung injury. J Surg Res 1992; 52: 656–662. [DOI] [PubMed] [Google Scholar]
- 13.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. J Parenteral Enteral Nutr 1983; 7: 582–586. [DOI] [PubMed] [Google Scholar]
- 14.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after abdominal trauma: a prospective randomized study. J Trauma 1986; 26: 874–881. [DOI] [PubMed] [Google Scholar]
- 15.Deitch EA. Bacterial translocation of the gut flora. J Trauma 1990; 30: S184–189. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery 1990; 107: 411–416. [PubMed] [Google Scholar]
- 17.Illig KA, Ryan CK, Hardy DJ, et al. Total parenteral nutrition induced changes in gut mucosal function: atrophy alone is not the issue. Surgery 1992; 112: 631–637. [PubMed] [Google Scholar]
- 18.Ephgrave K, Kleiman-Wexler R, Pfaller M. Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery 1993; 114: 815–821. [PubMed] [Google Scholar]
- 19.Shou J, Lappin J, Daly JM. Impairment of pulmonary macrophage function with total parenteral nutrition. Ann Surg 1994; 219: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 1995; 39: 44–52. [DOI] [PubMed] [Google Scholar]
- 21.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg 1997; 132: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediated intestinal cytokines. Ann Surg 1999; 229: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeWitt RC, Wu Y, Renegar KB, et al. Bombesin recovers gut-associated lymphoid tissue (GALT) and preserves immunity to bacterial pneumonia in TPN-fed mice. Ann Surg 2000; 231 (1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griensven M, Stalp M, Seekamp A. Ischemia-reperfusion directly increases pulmonary endothelial permeability in vitro. Shock 1999; 11: 259–263. [DOI] [PubMed] [Google Scholar]
- 25.Zallen G, Moore EE, Johnson J, et al. New mechanisms by which secretory phospholipase A2 stimulates neutrophils to provoke the release of cytotoxic agents. Arch Surg 1998; 133: 1229–1233. [DOI] [PubMed] [Google Scholar]
- 26.Walter B, Hagenlocker B, Ward P. Superoxide responses to formyl-methionyl-leucyl-phenylalanine in primed neutrophils. J Immunol 1991; 146: 3124–3131. [PubMed] [Google Scholar]
- 27.Koike K., Moore EE, Moore FA, et al. Gut phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia–reperfusion. Am J Physiol 1995; 268: G397–403. [DOI] [PubMed] [Google Scholar]
- 28.Koike K, Moore EE, Moore FA, et al. CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion. J Trauma 1995; 39: 23–28. [DOI] [PubMed] [Google Scholar]
- 29.Koike K, Yamamoto Y, Hori Y, et al. Group IIA phospholipase A2 mediates lung injury in intestinal ischemia–reperfusion. Ann Surg 2000; 232: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavez AM, Menconi MJ, Hodin RA, et al. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med 1999; 27: 2246–2251. [DOI] [PubMed] [Google Scholar]
- 31.Horie Y, Wolf R, Russell J, et al. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology 1997; 26: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 32.Kokura S, Wolf RE, Yoshikawa T, et al. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ Res 2000; 86: 205–213. [DOI] [PubMed] [Google Scholar]
- 33.Souza AL Jr, Poggetti RS, Fontes B, et al. Gut ischemia/reperfusion activates lung macrophages for tumor necrosis factor and hydrogen peroxide production. J Trauma 2000; 49: 232–236. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Wang X, Lasson A, et al. Roles of platelet-activating factor, interleukin-1β and interleukin-6 in intestinal barrier dysfunction induced by mesenteric arterial ischemia and reperfusion. J Surg Res 1999; 87: 90–100. [DOI] [PubMed] [Google Scholar]
- 35.Magnotti L, Upperman J, Xu D, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 1998; 228: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudsk KA, Minard G, Croce MA. A randomized trial of isonitrogenous enteral diets after severe trauma. Ann Surg 1996; 224: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houdijk APJ, Rijnsburger ER, Jansen J, et al. Randomized trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998; 352: 772–776. [DOI] [PubMed] [Google Scholar]