Abstract
Objective
To follow up in prospective fashion patients with coronary artery anastomoses completed endoscopically with robotic assistance. The robotic system was evaluated for safety and its effectiveness in completing microsurgical coronary anastomoses.
Summary Background Data
Recently there has been an interest in using robotics and computers to enhance the surgeon’s ability to perform endoscopic cardiac surgery. This interest has stemmed from the rapid advancement of technology and the desire to make cardiac surgery less invasive. Using traditional endoscopic instruments, it has not been possible to perform coronary surgery.
Methods
Nineteen patients underwent robotically assisted endoscopic coronary artery bypass grafting of the left internal thoracic artery (LITA) to the left anterior descending artery (LAD). Two robotic instruments and one endoscopic camera were placed through three 5-mm ports. A robotic system was used to construct the LITA–LAD anastomosis. All other required grafts were completed by conventional techniques.
Results
Seventeen LITA–LAD grafts (89%) had adequate intraoperative flow. The mean LITA–LAD graft flow was 38.5 ± 5 mL/min. At 8 weeks, LITA–LAD grafts were assessed by angiography and showed 100% patency with thrombolysis in myocardial infarction (TIMI) I flow. At a mean follow-up of 17 ± 4.2 months, all patients were NYHA class I and there were no adverse cardiac events.
Conclusions
The results from the first prospective clinical trial of robotically assisted endoscopic coronary bypass surgery in the United States showed favorable short-term outcomes with no adverse events. Robotic assistance is an enabling technology allowing the performance of endoscopic coronary anastomoses.
During the past 10 years, surgeons have begun to explore strategies to decrease the invasiveness of cardiac surgery. Great strides have been made in the development of beating heart surgery, which eliminates cardiopulmonary bypass. 1 Techniques also have been developed to decrease the size of the incision, and some procedures can now be performed through small thoracotomies 2 and limited sternotomies. The next step in the evolution of minimally invasive cardiac surgery would be the development of a completely endoscopic procedure. 3
The explosion of arthroscopic and laparoscopic techniques during the past several decades has revolutionized orthopedic and general surgery. However, endoscopic techniques have had little impact on microsurgical disciples, such as cardiac surgery. There are several reasons for this shortcoming. Traditional endoscopic instruments are not well suited for microsurgery 4 because of their length and the fulcrum effect. The length of the instruments magnifies even the smallest surgical tremor, making microsurgery difficult if not impossible. The fulcrum effect makes it difficult to judge precisely the deflection of the instrument tip for a prescribed movement of the instrument handle. Moreover, the nonintuitive movement of endoscopic tools (i.e., the instrument tip moving opposite to the handle) is problematic for fine microscopic suturing.
Traditional endoscopic visualization also is a hurdle to the performance of microsurgery. There is a lack of depth perception on the video image, and the hand-held endoscopic camera often yields an unsteady picture in highly magnified surgical fields. With recent advancements in computers 5 and robotic systems, it was hypothesized that robotics would prove to be an enabling technology for endoscopic cardiac surgery. 6,7 This study reports the 1-year follow-up of the first prospective, nonrandomized trial in North America of endoscopic coronary artery bypass grafting (ECABG).
The Zeus Robotic Surgical System (Computer Motion, Inc., Goleta, CA) was used in this trial. This system consisted of a surgeon console (Fig. 1), a computer control system, and three robotic arms (Fig. 2). The surgeon remained seated in a chair in front of the console, which consisted of a video monitor, two instrument handles, and a computer control system. The instrument handles were identical to traditional Castro-Viejo microsurgical needle drivers. As the surgeon moved the handles, his or her motions were mechanically relayed to a computer control system and digitized. Once the software processed the digital information (tremor filtration, motion scaling), the commands were relayed in real time to the two robotic arms. These arms, attached to the operating table, precisely controlled the instrument tips, which entered the patient through 5-mm ports. A third robotic arm held the endoscopic camera and was controlled by simple voice commands.

Figure 1. The Zeus Robotic Surgical System. The surgical console consists of a video monitor and two customized instrument handles. The surgeon grasps the handles and his or her movement is mechanically relayed to a computer control system, which digitizes the motion and relays it in real time to the robotic arms.
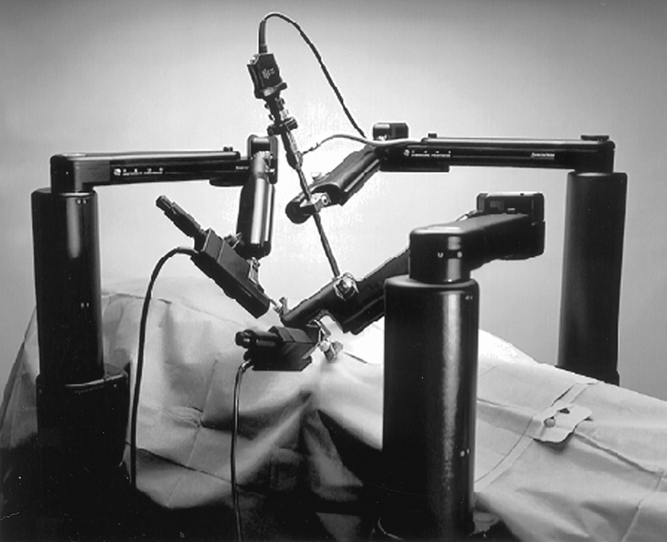
Figure 2. The robotic arms are attached to the operating table and precisely control two instrument tips that are positioned within the patient through 5-mm ports. A third voice-controlled arm is used to manipulate the endoscope.
Before beginning clinical studies, our group used the Zeus system to successfully complete coronary artery anastomoses in both cadaveric and live animals. 8,9 This single-center prospective clinical trial was approved by the Food & Drug Administration to evaluate the safety and efficacy of robotically assisted ECABG. All patients enrolled in this trial underwent robotically assisted anastomosis of the left internal thoracic artery (LITA) to the left anterior descending artery (LAD). All other grafts were sewn by hand in a traditional fashion. It was mandated that the procedures had to be performed on the stopped heart with cardiopulmonary bypass and cardioplegic arrest. The primary study end points were graft patency at 8 weeks and the incidence of device-related complications. The 12-month follow-up end points included major adverse cardiac events and recurrent angina.
METHODS
Patient Selection
After approval from our institutional review board, 28 patients were enrolled in the trial. Informed consent was obtained from each. Patients were considered eligible if they had angiographic evidence of occlusive (>50% stenosis) atherosclerotic disease of the LAD, required elective or urgent CABG surgery, and had had no previous cardiac surgery. Patients were excluded if they met one or more of the following criteria: age older than 80 years, ejection fraction of less than 40%, severe noncardiac conditions, severe peripheral vascular disease, myocardial infarction within 7 days of the procedure, need for concomitant or emergency surgery, or calcific or diffuse disease of the LAD, or if they were participating in other investigational device or drug studies.
Surgical Approach
All patients were placed supine, and arterial and venous access was obtained. After induction of general anesthesia, the three robotic arms of the Zeus system were attached to the operating table. The patient and the robotic arms were then prepared and draped in a sterile fashion. In 18 patients, a standard median sternotomy was performed and the LITA was taken down in a traditional manner. The pericardium was opened and the heart was exposed. The patients were heparinized and the activated clotting time was maintained at more than 450 seconds. The ascending aorta and right atrial appendage were cannulated and cardiopulmonary bypass was established. The patients were cooled to 32°C. A cannula was placed in the aortic root for antegrade administration of cardioplegic solution and a coronary sinus catheter was placed for retrograde cardioplegia administration.
In one patient, the LITA was harvested thoracoscopically through three endoscopic ports. The camera port was placed in the fourth intercostal space in the anterior axillary line and the two instrument ports were in the second and sixth intercostal spaces in the midaxillary line. A small (4-cm) anterior thoracotomy was made in the third intercostal space. This patient was placed on cardiopulmonary bypass with the Heartport system (Heartport, Inc., Redwood City, CA). 10
Robotic System Setup
The Zeus robotic arms were used to manipulate modified endoscopic instruments (Karl Storz, Culver City, CA). The arms were attached directly to the operating table. The surgeon controlled these arms by manipulating specially designed handles at the console. The handles allowed for four full ranges of motion (pan, roll, tilt, and in/out), as well as grasping. The surgeon’s motions were directly and precisely translated from the console to the robotic arms by a computer controller. Custom-designed software allowed for tremor elimination as well as motion scaling over a range of 2:1 to 10:1.
Three thoracoscopic ports were used to sew the anastomosis. This port placement was determined from our previous animal and cadaveric experience 11 and was optimal for the LAD anastomosis in our hands. The 5-mm right instrument port (Endopath, Ethicon, Inc., Somerville, NJ) was placed in the midline just below the xiphoid process. 12 The 5-mm camera port (Endopath, Ethicon) was positioned approximately 7 cm from the midline port in the fifth or sixth intercostal space, depending on the position of the LAD. The 5-mm left instrument port was placed 7 cm lateral to the camera port in the sixth or seventh intercostal space along the anterior axillary line. A 0° endoscope (Karl Storz) was used and attached to a three-chip video camera (Tricam SL, Karl Storz) and light source (Zenon 300, Karl Storz). The endoscope was manipulated with the Aesop (Computer Motion) voice-controlled robotic arm. The video was monitored both at the surgeon console and on a 21-inch monitor at the head of the operating table (Fig. 3). Specialized instrument tips were inserted into the ports.
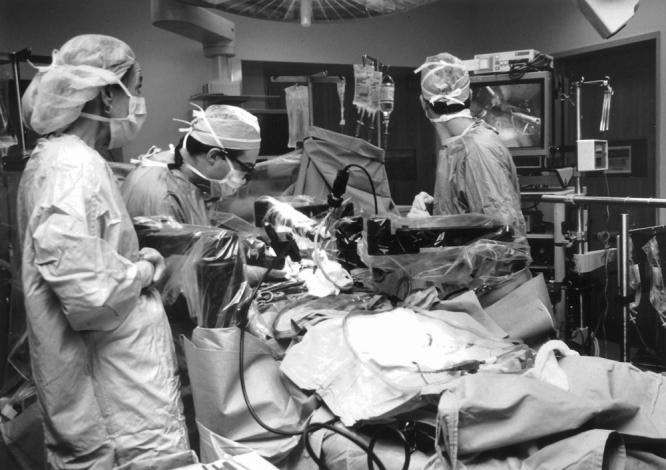
Figure 3. Intraoperative photograph of the operating table showing the robotic arms. A video monitor was positioned at the head of the table to allow the assistant to view the surgical field.
Cardioplegia Administration
In patients who required grafts in addition to the LITA–LAD, these grafts were performed first by traditional open chest methods. A cross-clamp was applied to the ascending aorta, and cold blood cardioplegic solution was administered in an antegrade fashion to achieve arrest. Myocardial temperature was measured in the intraventricular septum and maintained at less than 15°C. Additional cardioplegic solution was given as necessary throughout the remainder of the procedure in a retrograde fashion through the coronary sinus.
In patient 10, the Heartport Endoclamp device was used and cardioplegic solution was administered into the aortic root. Subsequently, cardioplegic solution was administered in a retrograde fashion through a percutaneously placed coronary sinus cannula.
Robotically Assisted Coronary Anastomosis
The heart was left in situ and was not suspended in a pericardial cradle. The chest retractor was relaxed to allow a more normal anatomical position of the chest wall. After cardioplegic arrest, an arteriotomy was made in the distal LAD. In five patients, this was performed with robotic assistance. In the remaining patients, the arteriotomy was performed manually. A continuous end-to-side anastomosis was performed endoscopically with the robotic instruments. The heel was sewn first and parachuted down, followed by completion of the toe of the anastomosis (Fig. 4). No manipulation of the heart was necessary to perform the anastomosis, and visualization of each stitch was excellent. An assistant held the LITA pedicle through the chest incision with standard instruments. The running anastomosis was performed with a specially designed 7-cm double-armed 7-0 polytetrafluoroethylene (Gore-Tex; W.L. Gore & Assoc., Newark, DE) suture. Motion scaling (2.5:1) was used for each anastomosis.
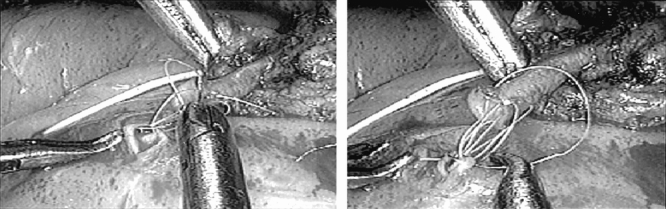
Figure 4. Intraoperative view of the anastomosis. The image was obtained with a 5-mm, 0° Storz endoscope positioned through a 5-mm port. The magnification is approximately 10 to 12×.
After completion of the grafts, the suture was tied endoscopically. No repair stitches were required. The clamp on the LITA and the aortic cross-clamp were removed, and the patients were rewarmed. The patients were then weaned from cardiopulmonary bypass. Protamine sulfate was administered to reverse the heparin, and the chest was closed in a standard fashion. No patient required inotropic support.
Intraoperative Graft Flow
Blood flow through the LITA graft was measured with a 2.0- to 2.5-mm ultrasonic flow probe and flowmeter (HT311, Transonic Systems, Inc., Ithaca, NY) and was recorded with Flow Trace 32 software (Transonic Systems). If flow was not judged to be adequate, selective coronary angiography was performed in the operating suite. The LITA graft was selectively catheterized via the left radial artery. An OEC model 9600 digital imaging system was used to visualize the anastomosis (Omega Electronics Corp., Salt Lake City, UT).
Follow-Up
During the postoperative period, the following data were collected: hemodynamic parameters, chest tube drainage, need for inotropic medications, length of intensive care unit stay, and length of hospital stay. Eight weeks after surgery, all patients underwent a second coronary angiogram of the LITA–LAD anastomosis to assess graft patency. There was 100% follow-up at 8 weeks. At late follow-up (17.0 ± 4.2 months), patients were contacted by phone and interviewed regarding their medical condition. A one-page questionnaire was used to inquire about recurrent cardiac symptoms or the occurrence of major adverse cardiac events. All 19 patients were available for follow-up (100%).
All procedures, including obtaining informed consent, were conducted in accord with the ethical standards of the Committee on Human Experimentation at the Pennsylvania State University.
RESULTS
Patient Demographics
Twenty-eight patients gave consent to participate in the study. Four (14%) were excluded before surgery for the following reasons (n = 1 each): preoperative myocardial infarction less than 7 days before surgery, preoperative work conflict, low ejection fraction, and hemodynamic instability. Five other patients (18%) were excluded during surgery because of a small or calcified LAD (n = 3), an innominate vein injury (n = 1), and inadvertent camera contamination (n = 1). A total of 19 patients completed the study, 15 men and 4 women. Their mean age was 59 ± 2 years. Their preoperative ejection fraction averaged 54% ± 3. Before surgery, all patients had class III or class IV New York Heart Association angina class.
Intraoperative Results
The operating room staff was able to assemble the robotic system safely and quickly. The average setup time was 16 ± 1 minutes (Table 1). There were no intraoperative complications related to the placement of the endoscopic ports. The robotic system functioned without problems in all 19 procedures. There were no intraoperative device-related complications.
Table 1. SURGICAL RESULTS
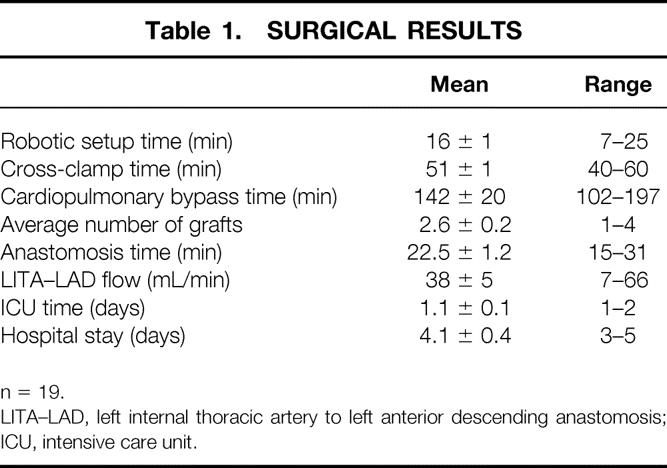
n = 19.
LITA–LAD, left internal thoracic artery to left anterior descending anastomosis; ICU, intensive care unit.
The average number of grafts performed was 2.6 ± 0.2 per patient. The mean cross-clamp time was 51 ± 1 minutes. The time required to perform the robotically assisted endoscopic LITA–LAD anastomosis was 22.5 ± 1.2 minutes (range 15–31). The last five anastomoses were all performed in less than 20 minutes. All robotically performed anastomoses were performed successfully, and no repair stitches were needed. The patient who had a minithoracotomy and cardiopulmonary bypass with the Heartport system had a cross-clamp time of 61 minutes and an anastomosis time of 28 minutes.
All patients underwent intraoperative ultrasonic determination of flow through the LITA graft. Seventeen grafts (89%) were patent and had excellent diastolic flow. The average graft flow was 38 ± 5 mL/min.
Two grafts had inadequate flow and were taken down and reconstructed manually. In one patient, an intraoperative coronary angiogram was performed. The entire LITA could not be visualized. However, the distal anastomosis was widely probe-patent. A tentative diagnosis of graft spasm was made. In the other patient, angiography was not available. Subsequent review of the videotape revealed a technical error, and this case represented a failure of surgical technique. Conversion to the manual technique was easily accomplished in both patients, and both left the operating room with patent LITA–LAD anastomoses.
Postoperative Course
There was only one postoperative complication. This patient required return to the operating room the evening of surgery for excessive mediastinal hemorrhage. The source of hemorrhage was identified as the lateral trocar insertion site. The bleeding was stopped successfully with electrocautery. No other complications were encountered in the postoperative period. The average length of stay in the intensive care unit was 1.1 ± 0.1 days. The average hospital stay was 4.1 ± 0.4 days.
Intermediate Follow-Up
Eight weeks after surgery, graft patency was assessed by coronary angiography. These studies were performed at the Pennsylvania State University. The angiograms were evaluated and read by an independent core laboratory at another institution. All LITA–LAD grafts were patent with TIMI 1 flow. Except for one graft that had a 50% stenosis at its insertion site, all grafts were free of stenoses.
Late Follow-Up
All 19 patients were available for late follow-up. The mean period of follow-up was 17 ± 4.2 months. There were no late complications. There were no reinterventions and no major adverse cardiac events in this group. All patients were asymptomatic, without recurrent angina.
DISCUSSION
This pilot clinical trial has documented the safety and efficiency of robotically assisted ECABG in a small group of patients with 1-year follow-up. These results suggest that robotics is an enabling technology that allows the performance of endoscopic cardiac surgery. Despite numerous efforts by groups around the world, hand-sewn endoscopic coronary anastomoses have not proven to be feasible. Robotic assistance clearly enhanced our surgical dexterity and allowed us to perform consistent endoscopic coronary anastomoses. 12 The robotic system addressed many of the physical limitations of traditional endoscopic surgery in the microsurgical setting. By allowing the surgeon’s motions to be translated into a digital format, it was possible to remove all tremor by filtering all high-frequency motion. The nonintuitive motion of endoscopic instruments could be eliminated with computer software because the instrument handle and tip were no longer attached to each other. In a similar manner, the fulcrum effect of the instrument at the port side was removed.
The voice-controlled robotic arm that controlled the endoscopic camera had the advantage of maintaining a stable image when compared with a manually held camera. 13,14 When performing surgery under high magnification, the slightest movement can distort the surgeon’s field. The robotic arm not only gave a steadier image but also allowed the camera to remain under the surgeon’s direct control. 15 All of these features of the robotic microsurgical system combined to help enable the surgeon to perform endoscopic anastomoses. 12
The principal shortcoming of this study was that it did not represent a completely endoscopic approach. Most of the patients had a sternotomy to allow manual performance of the other bypass grafts. However, our results show that the most difficult part of the procedure, the coronary anastomosis, can be performed endoscopically with robotic assistance. Moreover, it is the first report of 1-year follow-up of robotically assisted coronary anastomoses. It is encouraging that there have been no complications or major adverse cardiac effects in this period. However, it still represents short-term results, and it is obvious that continuing follow-up of this patient cohort will be important.
Early experience in Europe by Mohr et al 16 at the University of Leipzig, Reichenspurner et al 17 in Munich, and Schueler et al 18 in Dresden with ECABG has been very positive. The Dresden group has recently completed endoscopic robotically assisted surgery in 102 patients and has shown that it was possible to harvest the LITA and complete a LITA–LAD graft with excellent short-term results. 18 These early results with robotic systems suggest that a completely endoscopic approach to CABG is feasible in carefully selected patients. Although we believe that the complication rate will be significantly reduced 6 with this approach, randomized prospective multicenter trials are clearly needed to prove this hypothesis.
Another weakness of our study was that all patients underwent cardiopulmonary bypass and cardioplegic arrest. This is the gold standard for CABG around the world, but we believe that to be truly minimally invasive, ECABG will need to be performed on the beating heart. 3 Groups in London, Ontario, and Dresden have recently reported their early results with this approach. 19,20 The use of specially designed endoscopic intrathoracic stabilizers has enabled these procedures to be performed in the closed chest. These two groups have shown that this next exciting step, the ability to endoscopically construct a coronary anastomosis on the beating heart, is feasible, with excellent early graft patency.
Although dramatic progress has been made in the past 2 years, there is still a long way to go before there will be widespread adoption of robotically assisted ECABG. The procedure is now applicable only to carefully selected patients with single-vessel disease. There remain many challenges to the development of a reproducible, technically straightforward, and easy-to-perform procedure. Proper patient selection remains difficult and preoperative screening tests will need to be developed to define both appropriate candidates and correct port placement. The surgical choreography will need to be improved and streamlined to shorten the operating time. A major challenge will be the development of techniques to access vessels other than the LAD. This is critical because the great majority of patients referred for surgery have multivessel disease. Anastomotic devices may play a critical role in this area. We also need to explore new technology to enhance the operating space and increase the safety of working inside the limited thoracic space on the beating heart. 6 Finally, we must explore ways to make ECABG cost-effective and affordable. 21 This will require a close partnership between surgeons and industry.
In summary, our initial clinical experience has supported our hypothesis that robotic assistance is an enabling technology that allows the performance of ECABG. Graft patency at 2 months was excellent and 1-year clinical follow-up is encouraging. Further clinical trials are warranted to explore the potential of this exciting new technology and to establish its efficacy and clinical value.
Acknowledgments
The authors thank research nurses Vickie Eddinger and Mary Ellen May for their hard work and diligent efforts in collecting these data.
Discussion
Dr. William L. Holman (Birmingham, Alabama): Dr. Damiano’s report describes the use of an innovative surgical tool in patients with coronary artery disease. The potential for this technology to dramatically improve morbidity associated with a commonly performed cardiac operation, I think, is obvious. However, this is a preliminary experience. Important questions regarding cost and mortality relative to current surgical techniques as well as percutaneous techniques need to be addressed before we really understand how best to use robotically assisted surgery.
Ralph, can you give us an estimate of the training required to master robotically assisted techniques? Training is especially a concern for practicing cardiac surgeons who may not have extensive experience with endoscopic surgery. I note in your talk you mention 2 years for the FDA approval process and development of the technology. Clearly, that is not feasible, but I am not sure that a weekend course at a destination resort will be adequate either.
Second, I understand that the robotic system is quite expensive. Have you or the manufacturers of these devices projected the additional cost per case that is incurred when one uses the robotic system?
I look forward to your future reports on this exciting new technology and thank the Association for allowing me to discuss this paper.
Dr. Peter van Trigt (Greensboro, North Carolina): Dr. Damiano’s report of his impressive initial experience with robotically assisted endoscopic coronary bypass grafting provides us with carefully obtained information on this leading-edge technology in minimally invasive coronary surgery. He is to be congratulated for both the excellent results obtained with this new technology as well as the careful method in which he applied this new approach without sacrificing patient safety. This exciting report stimulates the imagination of how far the technology can be extended. In this initial series, all patients had left internal mammary artery grafting to the left anterior descending using cardiopulmonary bypass. Will it be possible to perform this operation without cardiopulmonary bypass and in a closed chest approach with robotic takedown of the left IMA?
Grafting of the left anterior descending only for single-vessel disease comprises a very small percentage of patients undergoing coronary bypass grafting in current times. What are the possibilities of extending robotically assisted closed-chest coronary bypass surgery to the circumflex and right coronary circulations which are not on the anterior surface of the left ventricle?
Finally, this technology is being evaluated at U.S. centers in accordance with IRB guidelines for patient enrollment and under FDA approval of the manufacturer’s equipment. Could you comment on this new technology and how it is being evaluated in Europe and how you feel those results should be interpreted?
Dr. Damiano, thank you for sharing your exciting work in this new technology with the Association, and I appreciate the opportunity to discuss this exciting paper. Thank you.
Dr. Ralph J. Damiano, Jr. (St. Louis, Missouri): I’d like to thank the discussants for their kind and provocative comments. I think the first question is an excellent one, and that is regarding the learning curve. As I stated in the presentation, we spent almost 2 years doing animal and cadaveric work, and quite a bit of that time was actually on procedure development, so I don’t want to give the impression that this is a 2-year process to learn this technology. And there are various new developments, particularly use of articulation of the instruments, that probably will shorten the learning curve.
The bottom line is that this is a very hot issue with the Food and Drug Administration and one that we have very little answers, not only for probably robotic technology but for most of the operations that we commonly perform in the operating room. I mean, what is the learning curve of learning to do a laparoscopic cholecystectomy? I mean, a lot of this stuff has not been well defined. We are, for the FDA, we are just about to start next month a prospective multicenter randomized trial with this, and any site is required to have at least 50 hours of training. And then the animal videos are going to be reviewed by a steering committee prior to proceeding for their first clinical case. And you can imagine, this is quite controversial.
I am very interested in learning curves, both with robotics and with endoscopic surgery. And at our institution we are right now in the midst of a prospective trial, looking at both medical students, residents, and faculty members, and putting them through a set of drills, both with laparoscopic instruments and with robotic technology, to try to define some of the aspects of surgical learning that are important. And perhaps next year I will have that data. I have excellent collaborators at Washington University, including Nat Soper and Mary Klingensmith has been involved in developing these protocols.
As far as the expense, that is also a critical issue, and we have not calculated at present the extra expense per case. I probably could give you a rough idea. The systems run anywhere from three quarters of a million for one of the systems to over $1 million for the other system. It is estimated that the disposable cost per case would be an additional $3,000 to $5,000. So, clearly, there are some economic issues involved with the adoption of this technology, but I would remind all the audience that this is computer-based technology, and we have seen the rapid, both increase in power of computers and rapidly decreasing cost. And I think we will probably see very similar trends in computer-assisted surgery in general.
The other question was, how do we avoid becoming the most expensive, least performed mid-cap procedure, since we can only do a single graft? That clearly is a big challenge to cardiac surgeons. I won’t minimize the challenges of doing multiple vessel grafting. A group in Dresden, Germany, has reported beating heart, double vessel grafting, but both have been on the anterior surface of the heart. I think we are going to have to develop new technology to help facilitate the anastomosis and, clearly, enhance the safety of these procedures, particularly on the beating heart, before we will be able to do multiple vessel grafting, and that is really the challenge for us in the next 5 years.
The final question was the European results. We have been held up, actually, by the FDA for a year since this initial pilot study before they approved the multicenter trial. And in Europe and Canada they have much less regulatory information. But, then again, the reports are really solely based on the individual centers’ accuracy in telling us the results. Now I will say that I have a great deal of respect for probably the two biggest centers in the world are in East Germany, the old East Germany, in Leipzig and Dresden, with excellent surgeons under the direction of Dr. Stefan Schueler and Dr. Frederick Mohr who have got a great deal of integrity, and I think both should be congratulated for very honestly presenting their results in quite a bit larger group of patients. But I think the verdict is still out, and I think we still have to show that these procedures will actually have a value for the patients and can be more widely applicable. But I think that there is a bright future in computer-assisted surgery. And as this clearly demonstrates, this does enhance surgical dexterity enough to allow us to perform procedures that truly are beyond our normal human capability. And I think certainly the future is exciting for the integration of computers into the operating room.
I’d like to thank the Association again.
References
- 1.Borst C, Grundeman PF. Minimally invasive coronary artery bypass grafting: an experimental perspective. Circulation 1999; 99: 1400–1403. [DOI] [PubMed] [Google Scholar]
- 2.Calafiore AM, DiGiammarco G, Teodori G, et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann Thorac Surg 1996; 611: 658–665. [DOI] [PubMed] [Google Scholar]
- 3.Damiano RJ. Endoscopic coronary artery bypass grafting: the first steps on a long journey. J Thorac Cardiovasc Surg 2000; 120: 806–807. [DOI] [PubMed] [Google Scholar]
- 4.Pagni S, Qaqish NK, Senior DG, Spence PA. Anastomotic complications in minimally invasive coronary bypass grafting. Ann Thorac Surg 1997; 63: S64–67. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Ruiz A, Smedira NG, Loop FD, et al. Robotic surgical instruments for dexterity enhancement in thoracoscopic coronary artery bypass graft. J Laparoendosc Adv Surg Tech 1997; 7: 277–283. [DOI] [PubMed] [Google Scholar]
- 6.Damiano RJ Jr, Reichenspurner H, Ducko CT. Endoscopic robotically-assisted coronary artery bypass grafting: present state-of-the-art and future directions. Adv Card Surg 2000; 12: 37–57. [PubMed] [Google Scholar]
- 7.Shennib H, Bastawisy A, Mack MJ, et al. Computer-assisted telemanipulation: an enabling technology for endoscopic coronary artery bypass. Ann Thorac Surg 1998; 66: 1060–1063. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson ER, Ducko CT, Sankholkar S, et al. Computer-assisted endoscopic coronary artery bypass grafting: a chronic animal study. Ann Thorac Surg 1999; 68: 838–843. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson ER Jr, Sankholkar S, Ducko CT, et al. Successful endoscopic coronary artery bypass grafting: an acute large animal trial. J Thorac Cardiovasc Surg 1998; 116: 1071–1073. [DOI] [PubMed] [Google Scholar]
- 10.Fann JI, Pompil MF, Burdeon TA, et al. Port access coronary artery bypass grafting. In: Cohen RG, Mack MJ, Fonger JD, Landreneau RJ, eds. Minimally invasive cardiac surgery. St Louis: Quality Medical Publishing; 1999: 181–187.
- 11.Ducko CT, Stephenson ER Jr, Sankholkar S, et al. Robotically-assisted coronary artery bypass surgery: moving toward a completely endoscopic procedure. Heart Surg Forum 1999; 2: 29–37. [PubMed] [Google Scholar]
- 12.Damiano RJ, Ehrman WJ, Ducko CT, et al. Initial United States clinical trial of robotically assisted endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2000; 119: 77–82. [DOI] [PubMed] [Google Scholar]
- 13.Kavoussi LR, Moore RG, Adams JB, et al. Comparison of robotic versus human laparoscopic camera control. J Urol 1995; 154: 2134–2136. [PubMed] [Google Scholar]
- 14.Geis WP, Kim HC, McAfee PC, et al. Synergistic benefits of combined technologies in complex, minimally invasive surgical procedures: clinical experience and educational processes. Surg Endosc 1996; 10: 1025–1028. [DOI] [PubMed] [Google Scholar]
- 15.Chitwood WR. Video-assisted and robotic mitral valve surgery: toward an endoscopic surgery. Semin Thorac Cardiovasc Surg 1999; 11: 194–205. [DOI] [PubMed] [Google Scholar]
- 16.Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999; 117: 1212–1214. [DOI] [PubMed] [Google Scholar]
- 17.Reichenspurner H, Damiano RJ, Mack M, et al. Experimental and first clinical use of the voice-controlled and computer-assisted surgical system Zeus for endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 1999; : 1181–1216. [DOI] [PubMed] [Google Scholar]
- 18.Cichon R, Kappert U, Schneider J, et al. The development of robotic enhanced endoscopic surgery for the treatment of coronary artery disease: experience in 102 patients. Circulation 2000; 102: 582. [DOI] [PubMed] [Google Scholar]
- 19.Kappert U, Cichon R, Schneider J, et al. Closed-chest coronary artery surgery on the beating heart with the use of a robotic system. J Thorac Cardiovasc Surg 2000; 120: 809–811. [DOI] [PubMed] [Google Scholar]
- 20.Boyd WD, Rayman R, Desai ND, et al. Closed-chest coronary artery bypass grafting on the beating heart with the use of a computer-enhanced surgical robotic system. J Thorac Cardiovasc Surg 2000; 120: 807–809. [DOI] [PubMed] [Google Scholar]
- 21.Doty JR, Fonger JD, Nicholson CF, et al. Cost analysis of current therapies for limited coronary artery revascularization. Circulation 1997; 96 (Suppl): II16–20. [PubMed] [Google Scholar]
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Dr. Ralph J. Damiano Jr., Chief, Cardiac Surgery, Division of Cardiothoracic Surgery, Campus Box 8234, Suite 3108 Queeny Tower, One Barnes-Jewish Hospital Plaza, St. Louis, MO 63110.
Accepted for publication December 2000.


