Abstract
Objective
To compare pancreas transplantation with systemic-enteric (SE) versus portal-enteric (PE) drainage in a prospective fashion.
Summary Background Data
To improve the physiology of pancreas transplantation, the authors developed a new technique of portal venous delivery of insulin and enteric drainage of the exocrine secretions.
Methods
During a 26-month period, the authors prospectively alternated 54 consecutive simultaneous kidney and pancreas transplants to either SE (n = 27) or PE (n = 27) drainage. The two groups were well matched for numerous characteristics. Maintenance immunosuppression in both groups consisted of tacrolimus, mycophenolate mofetil, and steroids.
Results
Patient survival rates were 93% SE versus 96% PE; kidney graft survival rates were 93% in both groups. Pancreas transplantation survival (complete insulin independence) was 74% after SE versus 85% after PE drainage with a mean follow-up of 17 months. The mean length of initial hospital stay was 12.4 days in the SE group and 12.8 days in the PE group. The SE group was characterized by a slight increase in the number of readmissions. The incidences of acute rejection (33%) and major infection (52%) were similar in both groups. The incidence of intraabdominal infection was slightly higher in the SE group. However, the early relaparotomy rate was similar between groups. The composite endpoint of no rejection, graft loss, or death was attained in 56% of SE versus 59% of PE patients.
Conclusions
These results suggest that simultaneous kidney and pancreas transplantation with SE or PE drainage can be performed with comparable short-term outcomes.
Vascularized pancreas transplantation was developed as a means to reestablish endogenous insulin secretion responsive to normal feedback controls. Pancreas transplantation is currently the only available form of autoregulating total endocrine replacement therapy that reliably establishes an insulin-independent euglycemic state and normal glucose homeostasis, resulting in the successful treatment of diabetes mellitus. With improvements in organ retrieval and preservation technology, refinements in diagnostic technology and surgical techniques, advances in clinical immunosuppression and antimicrobial prophylaxis, and experience in donor and recipient selection, success rates for pancreas transplantation have steadily increased. From 1966 through July 2000, more than 14,000 pancreas transplants were performed worldwide and reported to the International Pancreas Transplant Registry (IPTR). 1 In the United States, more than 1,200 pancreas transplants are performed annually, with 83% being simultaneous kidney and pancreas transplantations (SKPTs). The current 1-year actuarial patient, kidney, and pancreas (with complete insulin independence) graft survival rates after SKPT are 95%, 92%, and 84%, respectively. 1 With the advent of Medicare coverage, SKPT has become accepted as the preferred treatment option in selected patients with insulin-dependent diabetes mellitus and advanced nephropathy.
The history of clinical pancreas transplantation largely revolves around the development and application of various surgical techniques. According to IPTR data, most pancreas transplants are performed with systemic venous delivery of insulin and either bladder (systemic-bladder [SB]) or enteric (systemic-enteric [SE]) drainage of the exocrine secretions. 1,2 From 1988 through 1995, more than 90% of pancreas transplants were performed by the standard technique of SB drainage. 2 With an evolution in surgical techniques, a resurgence of interest has occurred in enteric exocrine drainage. Since 1995, the number of pancreas transplants performed with primary enteric drainage has steadily increased, accounting for 60% of cases in 1999. 1
Most pancreas transplants with enteric drainage are performed with systemic venous delivery of insulin (SE technique). 2 To improve the physiology of pancreas transplantation and to avoid the potential complications of systemic hyperinsulinemia (e.g., dyslipidemia, accelerated atherosclerosis, and insulin resistance), a new surgical technique was developed at our center using portal venous delivery of insulin and enteric drainage of the exocrine secretions (portal-enteric [PE]). 3,4 However, the proportion of cases with enteric exocrine drainage coupled with portal venous delivery of insulin has remained low and represents only 15% to 20% of enteric-drained pancreas transplants. 2 In the most recent IPTR analysis including pancreas transplants performed between 1996 to 1999, the 1-year pancreas graft survival rates were similar for PE versus SE drainage (83% and 84%, respectively). 1 The purpose of this study was to compare SKPT with SE versus PE drainage in a prospective fashion with standardized immunosuppression.
METHODS
Study Design
The pancreas transplantation program at the University of Tennessee, Memphis, was started in 1989. The first SKPT with PE drainage was performed in October 1990, and this patient continues to enjoy excellent dual allograft function more than 10 years later. From February 1997 through March 1998, we prospectively compared 32 consecutive pancreas transplantations performed with either SB or PE drainage. 5 During a 26-month period from April 1998 through May 2000, 54 consecutive SKPT recipients were entered into a prospective study of SE versus PE drainage at our center (Fig. 1). The technique to be performed was chosen before the transplant, with selection determined by an alternating methodology. In four patients, the preselected technique could not be performed because of intraoperative findings that favored one technique over the other. These four patients were included in the study, and subsequent pretransplant selection of technique continued on an alternating basis. A total of 27 patients were allocated to each technique.
Figure 1. Prospective study design comparing systemic-enteric (S-E) versus portal-enteric (P-E) drainage in simultaneous kidney and pancreas transplantation.
Organ Procurement, Preservation, and Preparation
The pancreas, kidney, or both were procured from heart-beating cadaveric donors in conjunction with multiple organ retrieval using standardized techniques. 6 University of Wisconsin (UW) solution was used for both in situ flush and storage of all organs under cold storage conditions. Whole organ pancreaticoduodenosplenectomy was performed by an en bloc technique. 6 Cold ischemia was minimized and pancreas preservation times were less than 24 hours in all cases and less than 12 hours in about one third of cases. 7 Before transplantation, the pancreas was reconstructed on the back table with a donor iliac artery bifurcation Y graft to the splenic and superior mesenteric arteries. 8,9 The PE procedure requires that the arterial bifurcation graft be constructed intentionally long for subsequent arterialization. The donor portal vein was mobilized and dissected back to the splenic and superior mesenteric venous confluence without the need for a venous extension graft. The proximal duodenal staple line (just distal to the pylorus) was inverted with suture, and the distal duodenal closure incorporated the third and a variable length of the fourth portion of the duodenum, as previously described. 3,4 The closure of the mesenteric root was reinforced with a running suture. The spleen was left attached to the tail of the pancreas to be used as a handle, but in some cases the splenic hilar structures were ligated in continuity before revascularization. The kidney was likewise prepared using standard techniques. The pancreaticoduodenal graft was repackaged separately and in sterile fashion in cold UW solution before implantation.
Recipient Selection and Surgical Procedure
Patients were selected for transplantation based on ABO blood type compatibility, period on the waiting list, and a negative T-lymphocytotoxic cross-match, in accordance with United Network for Organ Sharing guidelines. After preparation of the organ or organs, the recipient procedure was performed through a midline intraperitoneal approach. For SE cases, the whole organ pancreas was transplanted to the right common iliac vessels with end-to-side vascular anastomoses, followed by enteric drainage with either a diverting Roux limb or a side-to-side duodenoenterostomy. 8 The surgical technique of PE drainage has been previously described in detail by our group (Fig. 2). 4,8 The portal vein of the pancreas graft was anastomosed end to side to a major tributary of the superior mesenteric vein. The donor iliac artery bifurcation graft was brought through a window made in the distal ileal mesentery and anastomosed end to side to the right common iliac artery. The transplanted duodenum was anastomosed to a diverting Roux-en-Y limb of recipient jejunum. Splenectomy was performed after revascularization, and an attempt was made to anchor the tail of the pancreas to the anterior abdominal wall with interrupted sutures. These anchoring sutures permitted subsequent percutaneous, ultrasound-guided pancreas allograft biopsies to be performed as needed. 10
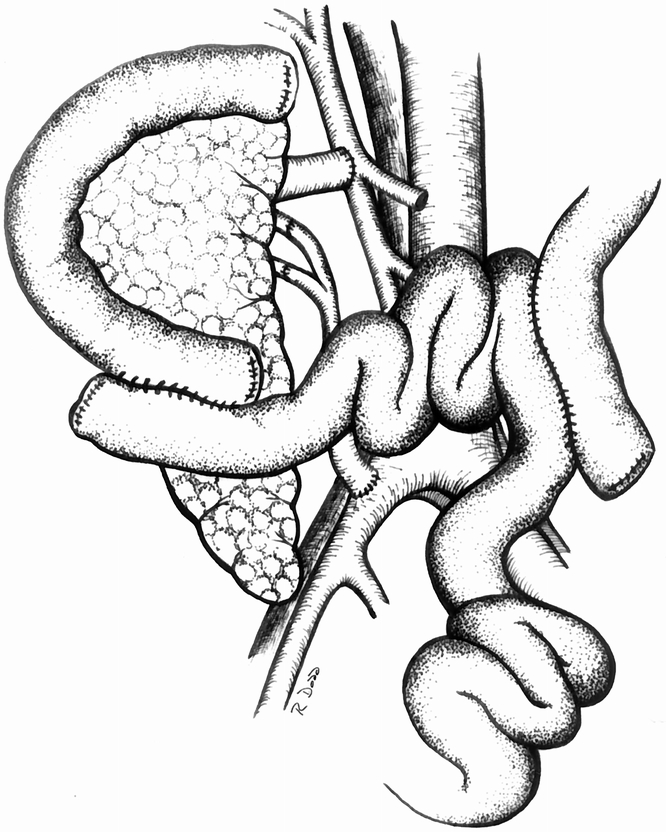
Figure 2. Technique of whole organ pancreaticoduodenal transplantation with portal venous drainage of insulin into a tributary of the superior mesenteric vein and primary enteric drainage of the exocrine secretions using a diverting Roux limb.
The kidney transplant was performed in the left iliac fossa, with end-to-side vascular anastomoses between the renal vessels and left external iliac vessels. After an extravesical ureteroneocystostomy by standard techniques, the renal allograft was then “retroperitonealized” by anchoring the sigmoid colon mesentery to the lateral peritoneal reflection with interrupted sutures.
A nasogastric tube, central venous line, and urethral catheter were placed at the beginning of the surgical procedure, and two closed-suction drains were placed medial and lateral to the pancreas allograft at the end of the procedure before wound closure.
Perioperative Management and Immunosuppression
Perioperative antibiotic prophylaxis consisted of a preoperative dose, an intraoperative dose, and three postoperative doses of cefazolin (1 g intravenous). All patients received single-strength sulfamethoxazole–trimethoprim 1 tablet per day for 6 to 12 months as prophylaxis for Pneumocystis pneumonia. Antifungal prophylaxis consisted of oral fluconazole 200 mg/day for 2 to 3 months. 11 Antiviral prophylaxis included intravenous ganciclovir (2.5–5 mg/kg twice daily) during the initial hospital stay, followed by oral ganciclovir (1 g 3 times daily) for 3 months (for 6 months if the donor was seropositive for cytomegalovirus and the recipient was seronegative). 12
All patients received primary immunosuppression with tacrolimus, mycophenolate mofetil (MMF), and steroids (Fig. 3). 13,14 Tacrolimus was started immediately after the transplant at a dosage of 0.1 to 0.2 mg/kg orally in two divided doses. Tacrolimus dosing was titrated to a 12-hour trough level of 15 to 20 ng/mL by immune monitoring (IMX) assay for the first 3 months. After 3 months, tacrolimus blood levels were maintained at 10 to 15 ng/mL in the absence of rejection. Oral MMF was begun immediately after the transplant at 2 g/day in two to four divided doses. The MMF dose was reduced in patients with gastrointestinal intolerance (nausea, vomiting, and diarrhea) or when the total white blood cell count was less than 3,000/mm3. MMF was discontinued temporarily in patients with active cytomegalovirus infection or septicemia, or when the total white blood cell count was less than 2,000/mm3; it was restarted later at a reduced dosage. Corticosteroids were administered as intravenous methylprednisolone 500 to 1,000 mg during surgery, followed by 250 mg on postoperative day 1, and then tapered to 30 mg/day oral prednisone by day 7. A gradual steroid taper was used, aiming at an oral prednisone dose of 15 mg/day at 3 months, 10 mg/day at 6 months, and 5 mg/day at 12 months.
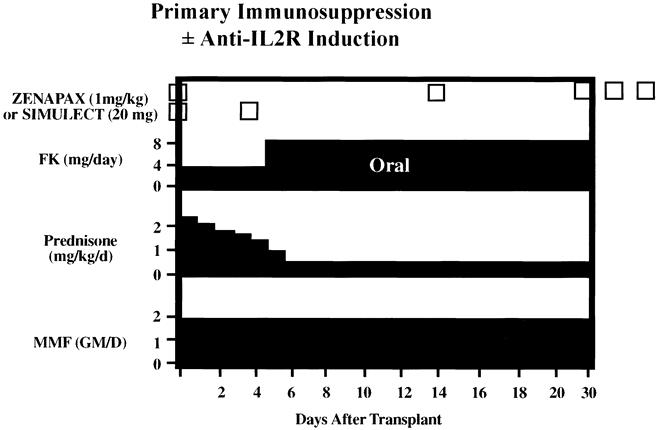
Figure 3. Immunosuppressive regimen with selected use of monoclonal antibodies directed against the interleukin-2 receptor (Zenapax or Simulect) as induction therapy. Maintenance immunosuppression is triple therapy with FK (tacrolimus), mycophenolate mofetil (MMF), and steroids.
Fewer than half of the patients received some form of antibody induction (Table 1). 15 Basiliximab 20 mg was administered intravenously over 15 to 30 minutes using a peripheral or central vein within 24 hours of transplant, and a second dose of basiliximab 20 mg was administered on postoperative day 4. Two dosing regimens of daclizumab (two doses or five doses) were used in this study. For the two-dose regimen, daclizumab 2 mg/kg was administered within 24 hours of the transplant and a second dose of daclizumab 2 mg/kg was administered intravenously over 15 to 30 minutes using a peripheral or central vein on postoperative day 14. For the five-dose regimen, daclizumab 1 mg/kg was administered intravenously over 15 to 30 minutes within 24 hours of transplant and then every 14 days (postoperative days 14, 28, 42, and 56) for a total of five doses. In two patients with acute tubular necrosis, thymoglobulin induction was initiated. Thymoglobulin was administered at 1.5 mg/kg per day through a central venous line over 4 to 6 hours as a continuous infusion. The intended duration of therapy was 5 to 7 days or until renal allograft function and was adjusted to maintain the total white blood cell count at more than 3,000/mm3 and the total platelet count at more than 100,000/mm3.
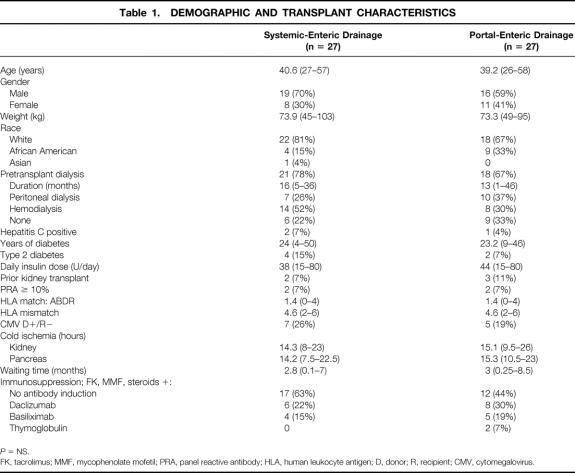
Table 1. DEMOGRAPHIC AND TRANSPLANT CHARACTERISTICS
P = NS.
FK, tacrolimus; MMF, mycophenolate mofetil; PRA, panel reactive antibody; HLA, human leukocyte antigen; D, donor; R, recipient; CMV, cytomegalovirus.
Antiplatelet therapy consisting of oral aspirin (81 mg/day) was administered to all patients. In addition, 2,000 to 3,000 units intravenous heparin was administered as a single dose during surgery before implantation of the pancreas. In selected patients, heparin prophylaxis was continued after surgery (continuous infusion of 300 units per hour for 24 hours, then 400 units per hour for 24 hours, and then 500 units per hour until postoperative day 5). Oral warfarin (Coumadin) in a single dose of 1 mg/day was administered to patients requiring prolonged vascular access or those with subsequent placement of a permanent central venous catheter.
Postoperative Monitoring
Patients were monitored in the intensive care unit for 24 to 36 hours before being transferred to the transplant unit. Nasogastric tube decompression was maintained for 2 to 3 days, closed-suction drainage for 3 to 5 days, and urethral catheter drainage for 5 to 7 days. After transplantation, duplex ultrasonography of the pancreas and kidney was performed on the first postoperative day and whenever clinically indicated. Recipients were serially monitored for daily fasting serum glucose, amylase and lipase levels, renal profiles, tacrolimus levels, and complete blood cell counts. Metabolic control and hormonal profiles were assessed by intravenous glucose tolerance testing, fasting and stimulated C-peptide levels, lipid profiles, and glycosylated hemoglobin levels. 8,16,17
The diagnosis of rejection was based on clinical criteria, 18 renal allograft dysfunction, serum amylase and lipase levels, 19 serum glucose levels, a change in the slope of glucose disappearance, 16 and renal or pancreas allograft histopathology. 10 Renal allograft rejection was suggested by an unexplained increase in the serum creatinine level of 0.3 mg/dL or greater and confirmed by ultrasound-guided percutaneous biopsy. Pancreas allograft rejection was suggested by an unexplained elevation in serum amylase, lipase, or glucose levels and confirmed by ultrasound-guided percutaneous biopsy. 10,16,18,19 The severity of rejection was defined according to the Banff criteria for kidney biopsies 20 and by a modification of the Maryland classification of allograft rejection for pancreas biopsies. 21 Mild renal allograft rejection was treated with intravenous methylprednisolone 500 to 1,000 mg/day for three doses. Antilymphocyte therapy with OKT3, ATGAM, or thymoglobulin for 5 to 10 days was used as the initial treatment for moderate or severe renal allograft rejection or for any pancreas allograft rejection. Steroid-resistant mild renal allograft rejection was also treated with antilymphocyte therapy.
Cytomegalovirus infection was defined as a positive blood culture, antigenemia, or IgM titer. 12 Invasive cytomegalovirus infection (cytomegaloviral disease) was defined as symptomatic cytomegalovirus infection or histologic evidence of tissue invasion. Treatment of cytomegaloviral infection consisted of intravenous ganciclovir for 2 to 4 weeks and a reduction in immunosuppression. Oral ganciclovir was given for a variable period after treatment of a documented cytomegaloviral infection. Other infections were recorded, with major infection defined as the need for hospital admission for diagnosis or treatment.
Statistical Analysis
Data are reported as mean and range. Minimum follow-up was 5 months (mean 17 months). Renal allograft loss was defined as death with function, transplant nephrectomy, and return to dialysis or to the pretransplant serum creatinine level. Pancreas graft loss was defined as death with function, transplant pancreatectomy, or the need for daily scheduled insulin therapy.
RESULTS
Over a 26-month period, 54 consecutive SKPT recipients were alternately assigned to either SE (n = 27) or PE (n = 27) drainage. Demographic, immunologic, and transplant characteristics of the groups are listed in Table 1. The groups were well matched for age, gender, weight, pretransplant dialysis status, duration of diabetes, transplant number, panel reactive antibody titer, human leukocyte antigen (HLA) match and mismatch, cytomegaloviral serologic status, kidney and pancreas cold ischemia, and time spent on the waiting list. The racial distribution differed slightly, with African American patients representing 15% of the SE group and 33% of the PE group. With regard to immunosuppression, 63% of SE patients and 44% of PE patients were managed with no antibody induction. The remaining SE patients received either daclizumab or basiliximab induction; the PE patients received daclizumab, basiliximab, or thymoglobulin in two patients with acute tubular necrosis.
Four patients (7%) were selected for one technique of transplantation and subsequently received the alternative technique because of technical considerations identified during surgery. Two patients chosen for PE drainage were switched to SE drainage because of obesity, with a thickened mesentery and small mesenteric vein identified during the dissection. In contrast, two patients selected for SE drainage were switched to PE drainage because of the presence of a previous kidney transplant in the right iliac fossa combined with severe right common iliac atherosclerosis. In this setting, the PE technique permitted transplantation of the pancreas outside the pelvis with arterial inflow based on either the distal aorta or left common iliac artery. These four crossover cases were included in the study.
Results are shown in Table 2. There were no significant differences in patient, kidney, or pancreas allograft survival rates after a mean follow-up of 17 months (Fig. 4). In the SE group, there were two deaths, one from severe hemolytic-uremic syndrome at 19 days, and the other from a cardiac event at 10.5 months after SKPT. In the PE group, there was one death from sepsis at 23 days after SKPT. There were two kidney graft losses in each group, including one death with function in each group. The remaining graft losses were due to hemolytic-uremic syndrome in the SE group and chronic rejection in the PE group. All but 3 of the 54 transplanted renal allografts had immediate function. Acute tubular necrosis, defined as the need for dialysis in the first week after transplant, occurred in one patient after SE and two patients after PE drainage. All three of these kidneys eventually functioned well.
Table 2. RESULTS
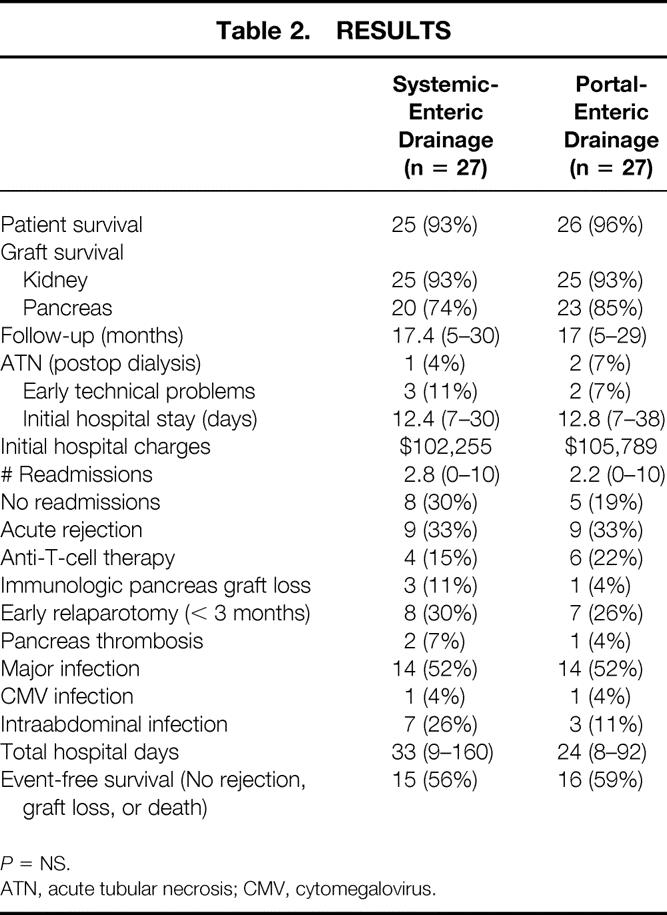
P = NS.
ATN, acute tubular necrosis; CMV, cytomegalovirus.
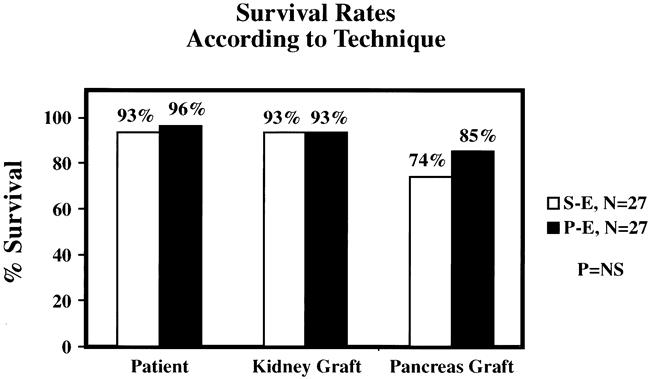
Figure 4. Patient, kidney, and pancreas graft survival rates according to surgical technique. No significant differences were noted.
Seven pancreas grafts were lost after SE drainage: two thrombosis, one pancreatectomy for persistent fistula, one death with function, and three resulting from chronic rejection. Four pancreas grafts were lost after PE drainage: thrombosis, pancreatectomy for persistent fistula, death with function, and chronic rejection (n = 1 each). All 54 transplanted pancreas allografts had initial function, although 3 were subsequently lost to thrombosis in the first week after transplant. The incidence of allograft pancreatitis, early leaks, or other technical problems related to the pancreas allograft was similar between groups.
The mean length of initial hospital stay was 12.4 days in the SE group and 12.8 days in the PE group (Fig. 5). Mean initial hospital charges were comparable between groups. The SE group was characterized by a slight increase in the number of readmissions (mean 2.8 SE vs. 2.2 PE) and total hospital days (mean 33 days SE vs. 24 days PE); neither difference was statistically significant. The incidence of acute rejection was similar (33%) in both groups, with immunologic pancreas graft loss occurring in three SE patients versus one PE patient (Fig. 6). The incidence of major infection was 52% in both groups, with one cytomegalovirus infection (4%) in each group. The incidence of intraabdominal infection was slightly greater in the SE group (26% SE vs. 11% PE), but the difference between groups was not significant. However, the early relaparotomy rate was similar between groups (30% SE vs. 26% PE).
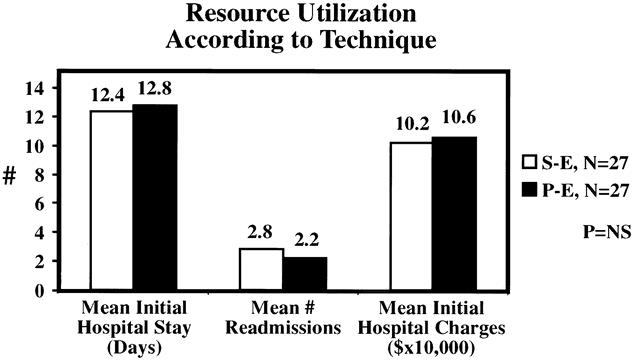
Figure 5. No significant differences were noted in surrogate markers of complications such as length of stay, readmissions, and hospital charges.
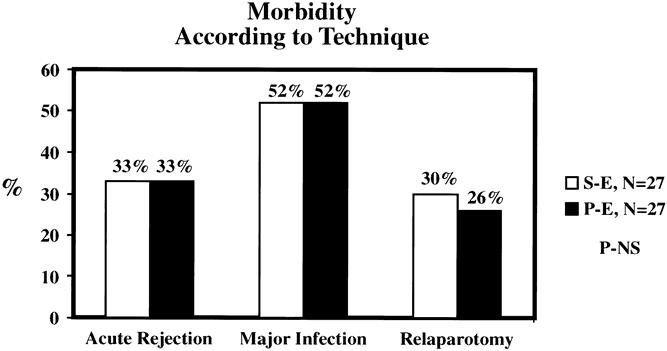
Figure 6. Immunologic, infectious, and surgical complication rates were comparable between groups.
Eight patients underwent early relaparotomy in the SE group: three for enteric leak, two for pancreas allograft thrombosis, two for small bowel obstruction, and one for a pancreatic fistula. In four of these patients, pancreas graft failure developed. In the PE group, seven patients underwent early relaparotomy: two for small bowel obstruction, two for bleeding, and one each for enteric leak, pancreas allograft thrombosis, and ureter leak. Of these seven, pancreas graft failure developed in two. The composite endpoint of no rejection, graft loss, or death was attained by 56% of SE and 59% of PE patients (Fig. 7).
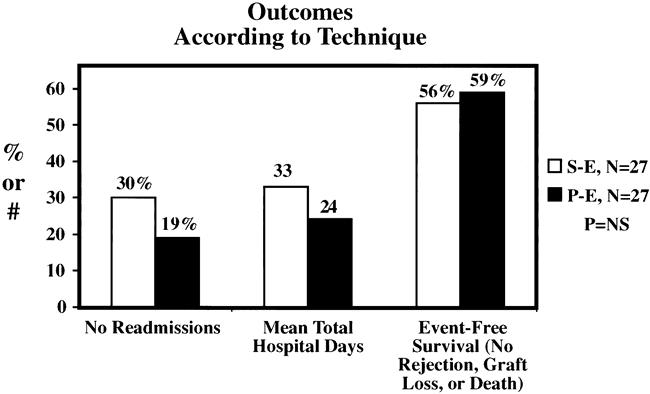
Figure 7. Hospital admission and event-free survival rates were comparable between groups.
DISCUSSION
Since the first SKPT in 1966, several techniques of pancreas transplantation have been studied. 22 From 1987 through 1995, most transplants were performed with SB drainage by the duodenal segment technique. 2 Although well tolerated in most recipients, SB drainage was associated with a finite and troublesome rate of unique metabolic and urologic complications resulting from altered physiology. 7,22,23 When these complications became intractable, conversion from bladder to enteric drainage (enteric conversion) was often necessary and successful. 22,23 Because of a favorable experience with enteric conversion, coupled with advances in preservation, donor selection, and immunosuppression that placed the duodenal segment at a lower risk for ischemic or immunologic injury, a resurgence of interest occurred in primary enteric drainage in an effort to avoid the complications of bladder drainage. Since 1995, the number of pancreas transplants performed with primary enteric drainage has steadily increased, accounting for 60% of cases in 1999. 1 In the past few years, the results of SKPT with enteric drainage have steadily improved and are now comparable to SKPT with bladder drainage. 1,2
Despite an evolution in surgical techniques, most pancreas transplants with enteric drainage are performed with systemic venous delivery of insulin. 2 To improve the physiology of pancreas transplantation and to avoid the potential complications of systemic hyperinsulinemia (e.g., dyslipidemia, accelerated atherosclerosis, and insulin resistance), a new surgical technique was developed at our center using portal venous delivery of insulin and enteric drainage of the exocrine secretion (PE drainage). 3,4 We have previously reported our initial experience with PE drainage, including both retrospective and prospective comparisons with control groups of patients who underwent SKPT with SB drainage. 5,8,24,25 According to IPTR data, however, the proportion of cases with PE drainage has remained low and represents only 15% to 20% of enteric-drained pancreas transplants. 2 In a recent survey of surgical techniques among pancreas transplantation centers, seven reported experience with the PE technique; five of them used a diverting Roux limb. 26 In the most recent IPTR analysis including pancreas transplants performed between 1996 to 1999, the 1-year pancreas graft survival rates were similar for PE versus SE drainage (83% and 84%, respectively). 1
Experience with pancreas transplantation with portal venous delivery of insulin dates back to the mid-1980s. Initial attempts involved segmental pancreas transplantation with gastric, 27 pyelic, 28 or jejunal 29,30 drainage. Whole organ pancreas transplantation using the PE technique was first described clinically by our group in 1992 3 and was based on experimental work by Shokouh-Amiri et al in a porcine model. 31–33 This new technique used a tributary of the superior mesenteric vein to reestablish portal venous drainage and differed substantially from other initial reports of whole organ pancreas transplantation with portal venous drainage. In 1990, Muhlbacher et al 34 described a technique involving an end-to-side anastomosis between the distal splenic vein of the donor and the recipient’s portal vein in combination with bladder drainage. In 1992, Rosenlof et al 35 applied Calne’s original technique to whole organ pancreas transplantation using an end-to-side anastomosis between the donor portal vein and recipient splenic vein coupled with enteric drainage. In each of these other series, however, the procedure was not widely applied because of technical problems associated with the vascular reconstruction. 36
In 1993, our group reported that PE pancreas transplantation with Roux limb diversion not only achieved acceptable metabolic control and eliminated hyperinsulinemia but was also associated with reduced postoperative complications. 24 In 1995, we compared 19 patients undergoing SKPT with the PE technique versus a retrospective control group of 28 patients receiving SKPT with the conventional SB technique. 25 Actuarial patient and graft survival rates at 1 and 3 years were no different in the two groups. Metabolic and urologic complications and urinary tract infections were more common in the SB group. Metabolic control was comparable between groups, and peripheral hyperinsulinemia did not occur in patients with PE drainage. In 1997, Nymann et al 37 from our group reported improved outcomes with increased experience with the PE technique. In 1998, Nymann et al 18 analyzed 47 SKPTs with graft function at 1 month, including 30 with SB and 17 with PE drainage. All patients had received cyclosporine-based therapy. Although the authors noted comparable patient and graft survival and surgical complication rates, the incidences of rejection and graft loss from rejection and the density of rejection were all lower in patients with PE drainage.
In 1995, Newell et al 38 from the University of Chicago reported their initial experience with a similar PE technique in 12 SKPT recipients compared with a retrospective matched control group of 12 SKPT patients with SB drainage. Six-month patient and graft survival rates were comparable, and the PE group had less acidosis, dehydration, hematuria, rejection, and need for enteric conversion. There were no differences in technical complications, and renal and pancreas allograft functions were similar. In 1996, Newell et al 39 presented 12-month follow-up on the same two groups with similar findings. In addition, the initial length of stay and total hospital days in the first year after transplantation were slightly lower in the PE group. There were no significant differences in costs and no delay in the diagnosis of rejection, and the authors concluded that their results confirmed the safety and efficacy of this new technique. In 1998, Bruce et al 40 from the University of Chicago reported their experience with 70 consecutive SKPTs with PE drainage performed between January 1992 and August 1997. They compared this group with a “historical” control group of 70 SKPTs with SB drainage performed between January 1987 and December 1994. One-year patient, kidney, and pancreas graft survival rates were comparable between groups. There were no significant differences in technical or immunologic graft failure rates, given that no enteric or anastomotic leaks were reported in this series. Renal and pancreas allograft functions at 1 year were similar. However, the total number of hospital days and surgical complications in the first year were significantly greater in the SB group, with the difference in these results almost entirely accounted for by a 21% rate of enteric conversion in patients with SB drainage. In addition, the authors noted a possible learning curve effect, with improved results in the latter 35 versus the former 35 SKPTs with PE drainage. Buell et al 41 later updated the University of Chicago experience, including 16 SKPTs with PE drainage without a Roux limb. This group reported good initial results with the PE technique in the absence of a diverting Roux limb.
In 1999, Reddy et al 42 reported a reduction in the surgical complication rate after pancreas transplantation with PE drainage that was attributed to increased experience with the technique. Also in 1999, Stratta et al 43 reported that the incidence of allograft pancreatectomy was not influenced by the surgical technique of implantation.
In 2000, Stratta et al 5 prospectively alternated 32 consecutive patients undergoing pancreas transplantation to SB (n = 16) or PE (n = 16) drainage with standardized immunosuppression. Patient and graft survival rates and surgical complications were comparable between groups after either SKPT or solitary pancreas transplantation. There were no graft losses resulting from either immunologic or infectious complications in either group, but the incidence of acute rejection was slightly greater in the SB group (44% SB vs. 31% PE); the difference was not significant. Moreover, the SB group was characterized by a slight increase in the number of readmissions, urinary tract infections, urologic complications, metabolic acidosis, and dehydration. Also in 2000, Cattral et al 44 prospectively studied 20 SKPTs with SB drainage followed by a sequential cohort of 20 consecutive SKPTs with PE drainage. One-year patient and graft survival rates were similar between groups. However, medical morbidity, cytomegalovirus infections, and acute rejection were more common in the SB group. Zibari et al 45 reported their initial experience with 17 SKPTs with PE drainage and a Roux-en-Y venting jejunostomy to monitor for rejection and prevent anastomotic leak. Patient, kidney, and pancreas graft survival rates were 100%, 100%, and 94%, respectively, after a mean follow-up of 16 months. In each of these studies, the authors concluded that SKPT with PE drainage could be performed with excellent short-term outcomes and manageable complications.
In 1999, Philosophe et al 46 from the University of Maryland reported their initial experience with 66 pancreas transplants with PE drainage compared with 183 pancreas transplants with SE drainage. Graft survival rates for SKPT, pancreas after kidney transplant (PAKT), and pancreas alone (PA) recipients were similar according to technique. However, when stratified for HLA matching, the incidence of rejection was lower in patients with PE drainage. In a follow-up report in 2000, Philosophe et al 47 compared 117 solitary pancreas transplants with PE drainage versus 70 with SE drainage. The authors noted not only an improvement in the pancreas graft survival rate, but also a decrease in the incidence and severity of rejection in patients with PE drainage. They concluded that PE drainage might be associated with an immunologic advantage.
In 2000, the Lyon group 48 reported a prospective study of 34 SKPT recipients randomized to either SE or PE drainage with a Roux limb. Patient and graft survival rates and complications were similar between groups. They concluded that whole organ pancreas transplantation with a standardized technique of PE drainage could be performed with short-term results comparable to the conventional technique of SE drainage.
Coincident with the Lyon study, we report here a prospective study of SE versus PE drainage in 54 consecutive SKPT recipients. The two groups were similar with respect to demographic, clinical, and immunologic variables; patient, kidney, and pancreas graft survival rates (including similar rates of graft loss from rejection and thrombosis); immunologic and surgical complications; surrogate markers such as length of stay and hospital charges; the ability to perform either the SE or PE technique; and the composite endpoint of event-free survival (no rejection, graft loss, or death). However, the two groups differed in that the SE group had a slight decrease in the pancreas graft survival rate, a slight increase in readmissions and total hospital days, and a slight increase in the incidence of intraabdominal infections. However, no significant differences in outcomes were noted.
In contrast to the results of previous studies, this prospective trial failed to show an immunologic advantage of PE drainage. 8,18,44,46,47 Although the incidence of acute rejection was identical between groups, the rate of pancreas graft loss from rejection was higher in the SE group. Moreover, one might contend that the PE group was immunologically at higher risk because of longer cold ischemia, an increased rate of acute tubular necrosis, more African American recipients, and fewer patients receiving antibody induction. We have recently shown that African American ethnicity is a risk factor for rejection in SKPT recipients. 49 Because of the confounding nature of these potential risk factors, it is premature to conclude that PE drainage does not have a salutary effect on antigen processing and delivery and the subsequent incidence of rejection.
Contrary to the general perception, the PE technique is not more technically demanding, nor is it associated with a higher incidence of surgical complications. Surgical time, blood loss, and ease of performance are similar when comparing pancreas transplantation with SB, SE, or PE drainage. In general, the venous anastomosis is easier to perform with the PE technique because access to and exposure of the mesenteric vein through a midline incision are simpler than approaching the iliac vein. However, the arterial reconstruction tends to be slightly more difficult because it requires a long Y graft tunneled through the distal ileal mesentery rather than a direct approach to the common iliac artery. Because the PE technique involves an intraabdominal approach, it may offer advantages in patients with previous pelvic surgery, pelvic transplants, or severe iliac atherosclerosis. However, obesity, thickened mesentery, and an inadequate mesenteric vein (<6 mm in diameter) are conditions that favor pancreas transplantation with either SB or SE drainage. Finally, the PE technique requires that a relatively long arterial bifurcation graft is available for use in the arterial reconstruction. In the absence of an adequate length of arterial graft, SB or SE drainage is performed preferentially without any added risk. In practice, the PE technique can be easily performed in nearly all cases in the absence of obesity.
In conclusion, these preliminary results suggest that SKPT with a standardized technique of PE drainage can be performed with short-term results comparable to the conventional technique of SE drainage. The PE technique does not appear to incur any additional or unique risks. Although studies by more centers with greater numbers of patients and longer follow-up are needed, both techniques should be included in the repertoire of pancreas transplantation. Because of its potential physiologic, metabolic, and immunologic advantages, SKPT with PE drainage may soon become the preferred technique of total endocrine replacement therapy.
Discussion
Dr. Richard J. Howard (Gainesville, Florida): Dr. Lawrence, Dr. Townsend, members, and guests. It is with great pleasure I rise to comment on the paper from Memphis, which has been a leader in pancreas and other transplantation techniques. This and the previous paper are largely technical papers.
Pancreas transplantation, as much as any other transplant procedure, has undergone an evolution over the last 30 years of operative techniques, trying to arrive at the optimal technique. We may be approaching that, but probably we are still not there yet. Much of the technical changes have centered around what do you do with all the enzymes that the pancreas secretes and how do you get rid of the enzyme drainage. Pancreas transplantation has gone from a variety of techniques. Bladder drainage was supposed to be the final solution. Now the pancreatic enzymes are put back into the intestine, which was used, interestingly, with the very first pancreas transplant done by Richard Lillehei in 1966. So we finally end up back where we started.
Now we have another question: what do you do with the blood? Traditionally, it has been put into the systemic circulation. Now we are going back to put it where it belongs, into the portal circulation. So I have some technical questions for the authors.
How do you decide when a patient is too obese or the mesentery is too thick in an obese patient to safely do this procedure into the portal drainage?
You suture the tail of the pancreas to the anterior abdominal wall to permit easy biopsy, and certainly being able to biopsy a pancreas is of great value in assessing rejection. Has this technique of suturing the tail of the pancreas to the anterior abdominal wall facilitated biopsy in all cases? Have you had any problems with hemorrhage, accidental biopsy of the intestine, or perforation of the intestine?
Another question is, why do you use induction therapy with antilymphocyte antibodies at the time of transplantation in some patients but not all? The majority of patients did not receive induction therapy.
Another question is, since the benefit of portal drainage seems to be as a trophic factor of insulin for the liver—certainly, Starzl has shown this in split-liver preparations in dogs—have you done any or do you contemplate doing any studies on the liver to see whether in fact there is an advantage of portal drainage of the venous outflow?
Based on the findings of this paper, there really was no difference between the two groups, and it comes as close to a randomized study as you could in having comparable groups between portal or systemic drainage. So since there was no difference outcome, which do you find easier and which would you rather do?
I thank the Society for the privilege of the floor.
Dr. Alan Hemming (Gainesville, Florida): Thank you, Mr. Chairman. I’d like to thank Dr. Gaber and his colleagues for the opportunity of discussing their paper on portal venous versus systemic venous drainage of the pancreas during simultaneous kidney and pancreas transplantation.
It is interesting to see how their approach to implanting the pancreas during transplantation has evolved over time. Some of the very first attempts at pancreas transplantation also utilized portal venous and enteric drainage. Surgeons, being relatively logical sorts, thought that placing the pancreas in the physiologic position should be advantageous. Unfortunately, early attempts met with high complication rates, both from enteric leaks and vascular complications. Since surgeons, being logical, also don’t like complications, the systemic venous and bladder technique was developed and pancreas transplantation went on to become successful, successful at least to maintaining glucose homeostasis, while at the same time a variety of metabolic complications unique to placing the pancreas in a nonphysiologic position arose.
Dealing with these complications has led to a renewed interest in implanting the pancreas in its physiologic position—that is, with portal venous drainage and enteric drainage.
The authors are to be congratulated on their excellent results. I have the following questions for the authors:
Once you have changed from bladder to enteric drainage, the majority of the metabolic complications are ameliorated. What, therefore, is the advantage to portal venous drainage? We know that the theory is that systemic hyperinsulinemia produced by systemic drainage is a bad thing. Has this in fact been borne out in practice?
The second question is, your study demonstrated no difference in acute rejection with portal venous drainage. Could you postulate as to why and offer your opinion of the advantage, if any, of portal venous antigen presentation?
Next question, or perhaps a comment: In performing portal venous drainage, we have found that it can be difficult to bring the iliac Y graft down to the recipient iliac artery, even though it is purposely constructed long. We have gone to routinely implanting an innominate artery interposition graft taken from the donor onto the recipient iliac prior to removing the pancreas from the colon. This has made the arterial anastomosis of the pancreas much easier, and we have had no vascular complications in the last 30 such performed. Have you had any difficulties in getting the artery to reach?
And, lastly, I echo Dr. Howard. You have demonstrated that your results with portal venous drainage are equivalent to systemic venous drainage. Which is currently your preferred method?
Thank you for the opportunity of discussing this paper.
Dr. Gazi B. Zibari (Shreveport, Louisiana): I, too, would like to thank the authors for providing me with the manuscript far in advance. I’m going to ask you three questions.
You performed a total of six simultaneous kidney/pancreas for type II diabetes mellitus, two in portal drainage and four in systemic drainage. How well did these patients do compared to the other type I diabetes patients?
There were four patients crossed over from one group to the other one because of technical problems. Were any of these patients type II diabetes secondary to morbid obesity and dissecting superior mesenteric vein?
There were a total of seven leaks that required early laparotomy. As we presented our data, the incidence of leak immediately postoperatively in our series was much smaller. Do you think that venting jejunostomy, decompressing this anastomosis, would be of some benefit?
And since you converted me 5 years ago to perform all bladder drainage into portal-enteric, I wonder whether or not you would be willing to consider this, especially for solitary pancreatic transplantation.
I thank you very much for privilege of the floor.
Dr. A. Osama Gaber (Memphis, Tennessee): Thank you very much. I wish to thank all the discussants for the excellent points that you have made.
I think that the technique of pancreas transplantation has truly evolved, and I didn’t think, as I presented my first paper about doing the portal-enteric pancreas transplantation to this Society in 1992, I never thought that I would be standing here to follow on a paper that shows modification of the technique that I thought was going to modify all the previous techniques. But I guess evolution is something that is very good.
The discussants, all of them, bring out some really important points related to this operation, portal-enteric transplantation. I’d like to say that it is very important, one of the major things that Dr. Amiri and myself have thought when we first devised this was not just the bladder complications, because that was actually—you could eliminate that by systemic-enteric—switching from systemic-bladder to systemic-enteric. But, actually, the metabolic complications were very important for us because of hyperinsulinemia.
And I would like to start by answering that first, because it came through in several discussions. We did show that there is quite significant evidence that when you put the pancreas in the portal vein that you do get two things that you do not get when you put it in the systemic circulation. One of them is significant alteration in the patterns of lipoprotein abnormalities that are associated with the development of atherosclerosis. I am not an expert in that, but my lipoprotein expert shows that lipoprotein A and several of the enzymes that are responsible for the position of the cholesterol in the blood vessels was unaltered when we delivered the insulin into the portal circulation. So that’s number one. Now, clearly, this study didn’t address that, and I think that is something that we should address in future studies.
The other thing was the fact that when you relieve systemic hyperinsulinemia, you are supposed to get less hypertension, less obesity, and less heart disease. Clearly, we did not look at that in this study.
Now there are complicating factors related to the immune suppression, which in themselves give the hyperinsulinemia, and they give the hypertension, and so forth. But that needs to be deciphered in slightly larger trials.
Now I would like to answer some specific questions. How do we decide about the obesity as an important technical problem for the surgeon? Now all of us, when we do pancreas transplants, we know that really you have to have a vein that is wide enough that you can put in an anastomosis and make sure that it is going to stay open. And the majority of the patients with a thickened mesentery have smaller veins. The other issue is the distance between the vein and the top of the mesentery. You always have to allow for swelling of the pancreas after perfusion because of edema and pancreatitis, and you want to make sure that that is not going to tent on the portal vein anastomosis and cause constriction.
Dr. Zibari said he would rather deal with bleeding than with pancreatic thrombosis, and I would add to that that I would rather deal with putting the pancreas anywhere where it works rather than have a thrombosis. There is a question. I would just—I mean, it is not a matter of proving that this is the best procedure in the world. You want the patient to have a functioning pancreas.
We do attempt to suture the tail of the pancreas to the anterior abdominal wall to get biopsies. We have actually published the first biopsy series in the world by doing percutaneous pancreatic biopsies. And that has not resulted in a lot of complication.
I think what we now try to do is if the pancreas sits where the stitch is going to really hold it for the biopsy, we do it. Otherwise, we are so good now at localizing it by ultrasonography that we do that, and our complication rate from biopsy has actually been extremely low. I think we have had maybe two episodes, one of bleeding and one of pancreatitis following the biopsy. Neither of them required a reoperation. So that, I think, we have done very well with that.
Now why did we use induction therapy in some and not other patients? It is related to the fact that in the middle of this study we started another immunosuppression study using induction. So that is why some of the patients had induction therapy and some not.
We did not do any work on the benefits of insulin as a trophic factor in the liver, and I think I have been asked that question repeatedly to the extent that it is time to do that study. And we will design something to do that.
The Y-graft idea that Dr. Hemming has raised is really a good idea, and I know that a lot of people in Maryland and the University of Chicago have done that. We have not had to do that. We had one case where Dr. Amiri and I one time had really a short vessel. And what we ended up doing was something sort of spur of the moment: we ligated the internal iliac artery, and that allowed us to mobilize the common iliac a lot higher, and so we didn’t need to use a Y graft. That was our solution.
The type II diabetic patients did just as well as the type I diabetic patients. Actually, they were not the ones where we had to switch technique. They were not part of that.
Now the reason why we didn’t show a difference in the rejection even though we were the first to report on the fact that you got low rejection, I think, is related to two factors. Number one, we had more high risk patients in the portal-enteric group. There were more black patients. There were more patients who had retransplant for some reason or another. So I think that is one reason. The other reason is that we have moved to very significant, very strong immunosuppression with tacrolimus mycophenolate and 50% of the patients get induction. But I think that when we use cyclosporine, the chance of rejection was higher, so you could see the difference. And now we have sort of obliterated that by the strong immunosuppression.
Once more, I would like to thank the Association for the privilege of presenting our paper here. I’d like to thank all the discussants.
Footnotes
Presented at the 112th Annual Session of the Southern Surgical Association, Dec. 4-6, 2000, Palm Beach, Florida.
Correspondence: Robert J. Stratta, MD, Department of Surgery, University of Tennessee, Memphis, 956 Court Ave., Suite A202, Memphis, TN 38163-2116.
E-mail: rstratta@utmem.edu
Accepted for publication December 2000.
References
- 1.Sutherland DER, Gruessner AC. International Pancreas Transplant Registry update. IPTR Newsletter 2000; 12: 1–23. [Google Scholar]
- 2.Gruessner AC, Sutherland DER. Analyses of pancreas transplant outcomes for United States cases reported to the United Network for Organ Sharing (UNOS) and non-US cases reported to the International Pancreas Transplant Registry (IPTR). In: Cecka JM, Terasaki PI, eds. Clinical transplants 1999. Los Angeles, CA: UCLA Immunogenetics Center; 2000: 51–69. [PubMed]
- 3.Shokouh-Amiri MH, Gaber AO, Gaber LW, et al. Pancreas transplantation with portal venous drainage and enteric exocrine diversion: a new technique. Transplant Proc 1992; 24: 776–777. [PubMed] [Google Scholar]
- 4.Gaber AO, Shokouh-Amiri H, Grewal HP, et al. A technique for portal pancreatic transplantation with enteric drainage. Surg Gynecol Obstet 1993; 177: 417–419. [PubMed] [Google Scholar]
- 5.Stratta RJ, Gaber AO, Shokouh-Amiri MH, et al. A prospective comparison of systemic-bladder versus portal-enteric drainage in vascularized pancreas transplantation. Surgery 2000; 127: 217–226. [DOI] [PubMed] [Google Scholar]
- 6.Stratta RJ, Taylor RJ, Spees EC, et al. Refinements in cadaveric pancreas–kidney procurement and preservation. Transplant Proc 1991; 23: 2320–2322. [PubMed] [Google Scholar]
- 7.Grewal HP, Garland L, Novak K, et al. Risk factors for post-implantation pancreatitis and pancreatic thrombosis in pancreas transplant recipients. Transplantation 1993; 56: 609–612. [DOI] [PubMed] [Google Scholar]
- 8.Stratta RJ, Gaber AO, Shokouh-Amiri MH, et al. Experience with portal-enteric pancreas transplant at the University of Tennessee-Memphis. In: Cecka JM, Terasaki PI, eds. Clinical transplant 1998. Los Angeles, CA: UCLA Tissue Typing Laboratory; 1999: 239–253. [PubMed]
- 9.Gill IS, Sindhi R, Jerius JT, et al. Bench reconstruction of pancreas for transplantation: experience with 192 cases. Clin Transplant 1997; 11: 104–109. [PubMed] [Google Scholar]
- 10.Gaber AO, Gaber LW, Shokouh-Amiri MH, et al. Percutaneous biopsy of pancreas transplants. Transplantation 1992; 54: 548–550. [DOI] [PubMed] [Google Scholar]
- 11.Stratta RJ. Ganciclovir/acyclovir and fluconazole prophylaxis after simultaneous kidney–pancreas transplantation. Transplant Proc 1998; 30: 262. [DOI] [PubMed] [Google Scholar]
- 12.Lo A, Stratta RJ, Egidi MF, et al. Patterns of cytomegalovirus infection in simultaneous kidney–pancreas transplant recipients receiving tacrolimus, mycophenolate mofetil, and prednisone with ganciclovir prophylaxis. Transplant Infect Dis 2001 3: 8–15. [DOI] [PubMed] [Google Scholar]
- 13.Reddy KS, Stratta RJ, Shokouh-Amiri H, et al. Simultaneous kidney–pancreas transplantation without anti-lymphocyte induction. Transplantation 2000; 69: 49–54. [DOI] [PubMed] [Google Scholar]
- 14.Stratta RJ, Gaber AO, Shokouh-Amiri MH, et al. Evolution in pancreas transplantation techniques: simultaneous kidney–pancreas transplantation using portal-enteric drainage without anti-lymphocyte induction. Ann Surg 1999; 229: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo A, Stratta RJ, Alloway RR, et al. Limited benefits of induction with monoclonal antibody to interleukin-2 receptor in combination with tacrolimus, mycophenolate mofetil, and steroids in simultaneous kidney-pancreas transplantation. Transplant Proc 2001 33: 1701–1703. [DOI] [PubMed] [Google Scholar]
- 16.Elmer DS, Hathaway DK, Abdulkarim AB, et al. Use of glucose disappearance rates (Kg) to monitor endocrine function of pancreas allografts. Clin Transplant 1998; 12: 56–64. [PubMed] [Google Scholar]
- 17.Hughes TA, Gaber AO, Shokouh-Amiri H, et al. Kidney–pancreas transplantation: the effect of portal versus systemic venous drainage of the pancreas on the lipoprotein composition. Transplantation 1995; 60: 1406–1412. [PubMed] [Google Scholar]
- 18.Nymann T, Hathaway DK, Shokouh-Amiri MH, et al. Patterns of acute rejection in portal-enteric versus systemic-bladder pancreas–kidney transplantation. Clin Transplant 1998; 12: 175–183. [PubMed] [Google Scholar]
- 19.Sugitani A, Egidi MF, Gritsch HA, et al. Serum lipase as a marker for pancreatic allograft rejection. Transplant Proc 1998; 30: 645. [DOI] [PubMed] [Google Scholar]
- 20.Solez K, Axelsen RA, Benediksson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993; 44: 411–424. [DOI] [PubMed] [Google Scholar]
- 21.Kuo PC, Johnson LB, Schweitzer EJ, et al. Solitary pancreas allografts: the role of percutaneous biopsy and standardized histologic grading of rejection. Arch Surg 1997; 132: 52–57. [DOI] [PubMed] [Google Scholar]
- 22.Stratta RJ, Taylor RJ, Gill IS. Pancreas transplantation: a managed cure approach to diabetes. Curr Prob Surgery 1996; 33: 709–816. [DOI] [PubMed] [Google Scholar]
- 23.Sindhi R, Stratta RJ, Lowell JA, et al. Experience with enteric conversion after pancreatic transplantation with bladder drainage. J Am Coll Surg 1997; 184: 281–289. [PubMed] [Google Scholar]
- 24.Gaber AO, Shokouh-Amiri H, Hathaway DK, et al. Pancreas transplantation with portal venous and enteric drainage eliminates hyperinsulinemia and reduces post-operative complications. Transplant Proc 1993; 25: 1176–1178. [PubMed] [Google Scholar]
- 25.Gaber AO, Shokouh-Amiri MH, Hathaway DK, et al. Results of pancreas transplantation with portal venous and enteric drainage. Ann Surg 1995; 221: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Carlo V, Castoldi R, Cristallo M, et al. Techniques of pancreas transplantation through the world: an IPITA center survey. Transplant Proc 1998; 30: 231–241. [DOI] [PubMed] [Google Scholar]
- 27.Calne RY. Paratopic segmental pancreas grafting: a technique with portal venous drainage. Lancet 1984; 1: 595–597. [DOI] [PubMed] [Google Scholar]
- 28.Gil-Vernet J, Fernandez-Cruz L, Andreu J, et al. Clinical experience with pancreaticopyelostomy for exocrine pancreatic drainage and portal venous drainage in pancreas transplantation. Transplant Proc 1985; 17: 342–345. [Google Scholar]
- 29.Tyden G, Wilczek H, Lundgren G, et al. Experience with 21 intraperitoneal segmental pancreatic transplants with enteric or gastric exocrine diversion in humans. Transplant Proc 1985; 17: 331–335. [Google Scholar]
- 30.Sutherland DER, Goetz FC, Moudry KC, et al. Use of recipient mesenteric vessels for revascularization of segmental pancreas grafts: technical and metabolic considerations. Transplant Proc 1987; 19: 2300–2304. [PubMed] [Google Scholar]
- 31.Shokouh-Amiri MH, Rahimi-Saber S, Andersen AJ. Segmental pancreatic autotransplantation in the pig. Transplantation 1989; 47: 42–44. [DOI] [PubMed] [Google Scholar]
- 32.Shokouh-Amiri MH, Falholt K, Holst JJ, et al. Pancreas endocrine function in pigs after segmental pancreas autotransplantation with either systemic or portal venous drainage. Transplant Proc 1992; 24: 799–800. [PubMed] [Google Scholar]
- 33.Shokouh-Amiri MH, Rahimi-Saber S, Andersen HO, et al. Pancreas autotransplantation in pig with systemic or portal venous drainage: effect on the endocrine pancreatic function after transplantation. Transplantation 1996; 61: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 34.Muhlbacher F, Gnant MFX, Auinger M, et al. Pancreatic venous drainage to the portal vein: a new method in human pancreas transplantation. Transplant Proc 1990; 22: 636–637. [PubMed] [Google Scholar]
- 35.Rosenlof LK, Earnhardt RC, Pruett TL, et al. Pancreas transplantation: an initial experience with systemic and portal drainage of pancreatic allografts. Ann Surg 1992; 215: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees M, Brons IGM. Pancreas transplantation: a historical perspective from a single institution. In: Hakim NS, Stratta RJ, Dubernard JM, eds. Second British Symposium on Pancreatic Transplantation. London, UK: Royal Society of Medicine Press; 1998: 85–89.
- 37.Nymann T, Elmer DS, Shokouh-Amiri MH, et al. Improved outcome of patients with portal-enteric pancreas transplantation. Transplant Proc 1997; 29: 637–638. [DOI] [PubMed] [Google Scholar]
- 38.Newell KA, Woodle ES, Millis JM, et al. Pancreas transplantation with portal venous drainage and enteric exocrine drainage offers early [PubMed]
- 39.Newell KA, Bruce DS, Cronin DC, et al. Comparison of pancreas transplantation with portal venous and enteric exocrine drainage to the standard technique utilizing bladder drainage of exocrine secretions. Transplantation 1996; 62: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 40.Bruce DS, Newell KA, Woodle ES, et al. Synchronous pancreas–kidney transplantation with portal venous and enteric exocrine drainage: outcome in 70 consecutive cases. Transplant Proc 1998; 30: 270–271. [DOI] [PubMed] [Google Scholar]
- 41.Buell JF, Woodle ES, Siegel C, et al. Portal-enteric drained simultaneous pancreas-kidney transplantation: To Roux or not to Roux? [abstract]. Proceedings of the 7th World Congress of the International Pancreas and Islet Transplant Association. 1999; 57 (A18). [Google Scholar]
- 42.Reddy KS, Stratta RJ, Shokouh-Amiri MH, et al. Surgical complications after pancreas transplantation with portal-enteric drainage. J Am Coll Surg 1999; 189: 305–313. [DOI] [PubMed] [Google Scholar]
- 43.Stratta RJ, Gaber AO, Shokouh-Amiri MH, et al. Allograft pancreatectomy after pancreas transplantation with systemic-bladder versus portal-enteric drainage. Clin Transplant 1999; 13: 465–472. [DOI] [PubMed] [Google Scholar]
- 44.Cattral MS, Bigam DL, Hemming AW, et al. Portal venous and enteric exocrine drainage versus systemic venous and bladder exocrine drainage of pancreas grafts: clinical outcome of 40 consecutive transplant recipients. Ann Surg 2000; 232: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zibari GB, Aultman DF, Abreo KD, et al. Roux-en-Y venting jejunostomy in pancreatic transplantation: a novel approach to monitor rejection and prevent anastomotic leak. Clin Transplant 2000; 14: 380–385. [DOI] [PubMed] [Google Scholar]
- 46.Philosophe B, Taylor JP, Schweitzer EJ, et al. Portal venous drainage in pancreas transplantation: is there an immunologic advantage [abstract]? Proceedings of the 7th World Congress of the International Pancreas and Islet Transplant Association. 1999; 56 (A15). [Google Scholar]
- 47.Philosophe B, Farney AC, Schweitzer EJ, et al. The superiority of portal venous drainage over systemic venous drainage in solitary pancreas transplantation [abstract]. Proceedings of the 17th International Congress of the Transplantation Society. 2000; 115 (AO330). [Google Scholar]
- 48.Petruzzo P, Da Silva M, Feitosa LC, et al. Simultaneous pancreas–kidney transplantation: portal versus systemic venous drainage of the pancreas allografts. Clin Transplant 2000; 14: 287–291. [DOI] [PubMed] [Google Scholar]
- 49.Lo A, Stratta RJ, Egidi MF, et al. Outcomes of simultaneous kidney-pancreas transplantation in African-American recipients: a case-control study. Clin Transplant 2000; 14: 572–579. [DOI] [PubMed] [Google Scholar]