Abstract
Objective
To evaluate the efficacy of intraoperative ultrasound in obtaining adequate surgical margins in women undergoing lumpectomy for palpable breast cancer.
Summary Background Data
Adequacy of surgical margins is a subject of debate in the literature for women undergoing breast-conserving therapy. The emerging technology of intraoperative ultrasound-guided surgery lends itself well to a prospective study evaluating surgical accuracy and margin status after lumpectomy.
Methods
Two groups of women undergoing lumpectomy for palpable breast cancer were studied, one group using intraoperative ultrasound (n = 27) and the other without (n = 24). Pathologic specimens were evaluated for size, margins, and accuracy, and patients were questioned about satisfaction with cosmetic results.
Results
Surgical accuracy was improved with intraoperative ultrasound-guided surgery. Margin status was improved, patient satisfaction was equivalent, and cost was not affected using ultrasound technology. Intraoperative ultrasound appears especially efficacious for women whose preoperative mammogram shows dense parenchyma surrounding the lesion.
Conclusions
The use of ultrasound-guided surgery optimizes the surgeon’s ability to obtain satisfactory margins for breast-conserving techniques in patients with breast cancer. Patient satisfaction is excellent and a cost savings is most likely realized.
Although A-mode or non-real-time B-mode ultrasound imaging started in the 1960s, it was of limited clinical utility. With the introduction of high-frequency real-time B-mode ultrasound in the late 1970s, the surgeon could use ultrasound to guide surgical procedures. Special intraoperative probes were developed, and in the 1980s intraoperative ultrasound (IOUS) was developed for hepatobiliary surgery, neurosurgery, and vascular surgery. 1 Breast surgeons were quick to begin using office-based ultrasound for defining breast lesions and guiding needle biopsy of ultrasound-visible lesions, but the transfer of this technology to the surgical suite for breast procedures has been a recent phenomenon.
Breast-conserving therapy (BCT) has gained wide acceptance as providing long-term survival equal to that seen with mastectomy for early-stage breast cancers, and accordingly the number of lumpectomy procedures has increased dramatically. Too often, however, the surgeon is disappointed to discover that a lumpectomy performed for a small palpable tumor fails to achieve a complete excision with histopathologically negative margins. The patient may then undergo a second resection with the goal of obtaining clear pathologic margins. This recommendation for reexcision often occurs even as conflicting data are published about the need for such margins to be completely free of malignancy.
In 1999, Harlow et al 2 described the use of IOUS localization of nonpalpable lesions to guide surgical lumpectomy procedures in an effort to obtain clear margins. They reported an impressive overall success rate of 97% (63/65) in achieving pathologically negative excision margins with ultrasound guidance. Their results compared favorably with a 50% to 70% range of success typically reported for obtaining negative margins with BCT. 3
Localization of small palpable breast tumors using IOUS offers several potential benefits for the patient desiring BCT. We hypothesized that IOUS would provide the surgeon with more precision in localizing tumors than palpation alone. This would result in a lower positive-margin rate, thereby decreasing the frequency and expense of additional resection procedures. We further hypothesized that IOUS would improve cosmesis by enabling the surgeon to position the incision on the breast optimally and minimize the resection of normal breast parenchyma.
METHODS
Patient Selection
Fifty-one women with breast cancer were recruited for this study. All surgical procedures were performed between December 1, 1998, and October 15, 2000. Patients were randomized to receiving ultrasound-guided surgery (n = 27) or standard surgical excision without ultrasound (n = 24). Patients were chosen for the study if they had biopsy-proven infiltrating ductal carcinoma of the breast, stage T1 or T2. Only patients who had undergone preoperative core biopsy of the lesion were eligible.
Surgical Procedure
For patients receiving IOUS, lesions were localized before skin incision by a 7.5-mHz linear-array ultrasound probe (B&K Medical, Marlborough, MA). The probe was covered with a sterile sleeve and sterile gel was applied to the skin surface overlying the lesion. An initial transverse image was obtained, and the lesion was centered on the ultrasound screen (Fig. 1). A sterile skin marker was then used to mark the lesion along the transverse axis. The process was repeated in the cranial–caudal plane. The accuracy of this localization was reconfirmed before proceeding with incising the skin. The skin flaps were created with scissors, and IOUS was used in the open wound overlying the palpable mass to aid dissection to obtain excision of the tumor with the desired 1-cm margins of surrounding breast parenchyma. If the tumor location did not permit a clear 1-cm-deep margin, fascia overlying the pectoralis major was taken. A postexcision specimen ultrasound was performed immediately after the specimen was resected, with transverse and sagittal images obtained. If the margin appeared less than the 1-cm desired margin, additional breast parenchyma was resected in that direction and separately labeled and sent to pathology for evaluation. All specimens were oriented by sutures placed by the surgeon.
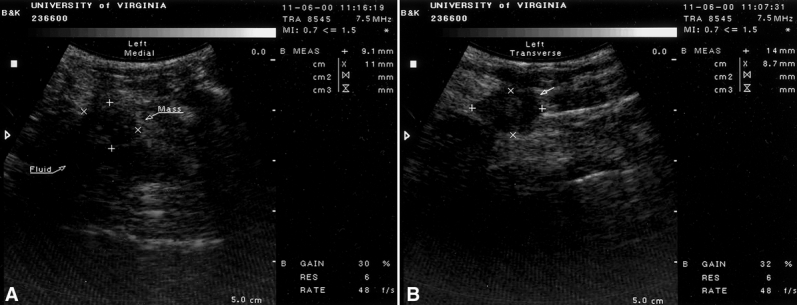
Figure 1. Two images in longitudinal (A) and transverse (B) planes of a palpable breast cancer. A 7.5-mHz probe is used on the skin to guide the surgical excision. Cursors delineate the margin.
Patients not receiving IOUS underwent a standard excision of their palpable masses. No sham ultrasound procedure was performed.
Data Analysis
Preoperative demographic data including age, family history, tumor stage, and mammographic interpretation were gathered. Intraoperative data including length of surgery, cost, and ultrasound findings were recorded. At 2 weeks after surgery, the patient was asked to fill out a questionnaire evaluating her satisfaction (1–5 scale) with the overall cosmetic result.
All data were compared between groups using standard statistical evaluations for means and standard error. Data were compared between groups using analysis of variance calculations. Analysis was performed using SPSS-MAC (SPSS, Inc., Chicago, IL). P < .05 signified statistical significance. Data are presented as means ± standard deviation.
Pathology
A positive margin was defined as any margin where tumor cells were microscopically visible on final histopathologic evaluation. Specimens with negative margins were categorized by location of and distance from the closest margin. One of the authors (L.C.) reviewed the margins of any specimen where the nearest margin could not be determined from the pathology report.
RESULTS
Demographic and Preoperative Features
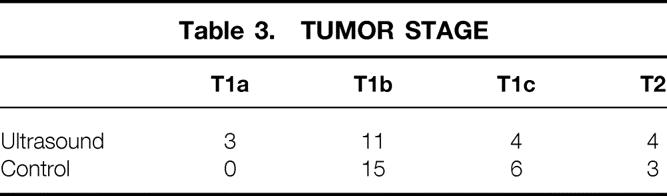
As shown in Table 1, patient demographics were similar in the two groups for age and family history. Six of 27 patients in the IOUS group were younger than 50 years; 5 of 24 patients in the conventional surgery group were younger than 50. At preoperative interviews designed to assess family history, 4 of 27 patients in the IOUS group and 6 of 24 in the conventional surgery group reported breast cancer in a single first-degree relative. No patient in either group reported having two first-degree relatives with breast cancer. In reviewing the preoperative mammograms on all patients, we found that 26 of 27 patients in the IOUS group had Breast Imaging Reporting and Data System (BI-RADS) IV or V tumors; all 24 patients in the conventional surgery group had mammograms judged BI-RADS IV or V (Table 2). Four patients in the IOUS group had tumors greater than 2 cm in diameter by mammographic evaluation (T2 lesion); 3 of the 24 patients in the conventional surgery group had T2 tumors (Table 3).
Table 1. PATIENT DEMOGRAPHICS
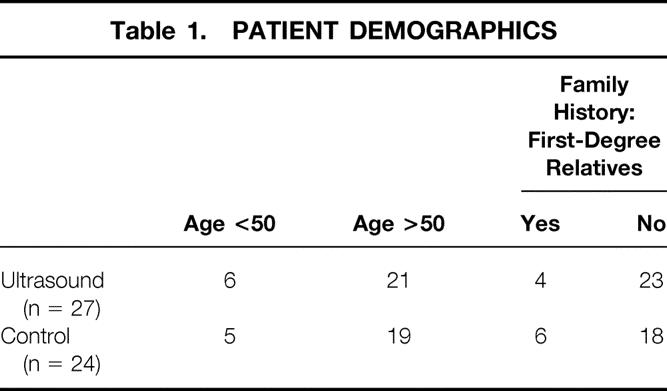
Table 2. BI-RADS CLASSIFICATION
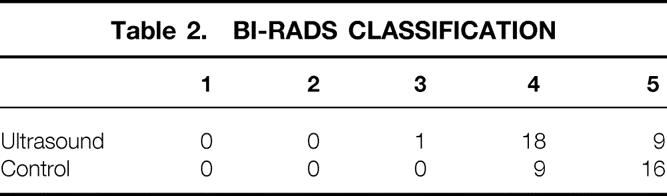
Table 3. TUMOR STAGE
Pathology
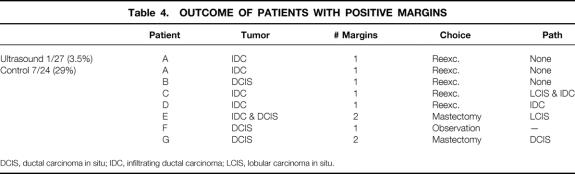
One of 27 (3.5%) patients in the IOUS group had a positive margin (infiltrating ductal carcinoma in a single margin;Table 4). This patient underwent reexcision and no residual disease was identified. Seven of 24 (29%) patients in the conservative surgery group had a positive margin (P < .05). Three patients had one or more margins for ductal carcinoma in situ only. Three of the patients had infiltrating ductal cancer at a single margin; one patient had both infiltrating ductal cancer at one margin and ductal carcinoma in situ at a second margin.
Table 4. OUTCOME OF PATIENTS WITH POSITIVE MARGINS
DCIS, ductal carcinoma in situ; IDC, infiltrating ductal carcinoma; LCIS, lobular carcinoma in situ.
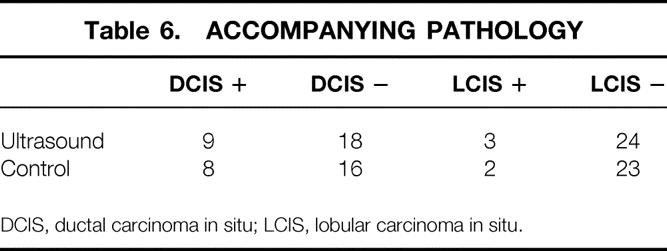
Fewer patients in the IOUS group had positive margins, and we also found that the margin of uninvolved breast tissue was greater (7.6 ± 2.0 mm vs. 4.8 ± 1.4 mm) in the IOUS patients compared with the controls. We found no difference between the two surgical groups in the location of the closest margin (Table 5). Likewise, we found no difference between the two groups in the number of patients with ductal carcinoma in situ or LCIS accompanying the excised primary tumor (Table 6).
Table 5. NEAREST MARGIN LOCATION
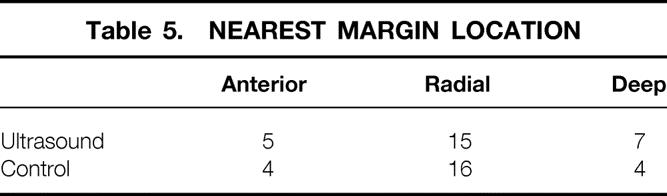
Table 6. ACCOMPANYING PATHOLOGY
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Specimen size was 104 ± 8 cm3 for the IOUS group versus 114 ± 5.6 cm3 for the control group (P = NS). Comparing specimen volume with the preoperative mammographically determined parenchymal density (Table 7), we found that patients with dense breasts had an increased volume of surgically removed tissue. A total of 24 patients had dense breasts on preoperative mammograms; 10 were randomized to the IOUS group and 14 to the control group. In these patients, the specimen volume was 127 cm3 in the IOUS group and 180 cm3 in the control group. If the breast tissue was not dense, the use of IOUS made little difference (90 vs. 102 cm3). Statistical significance was not reached in either group, however.
Table 7. SPECIMEN VOLUME VERSUS DENSITY
Outcome
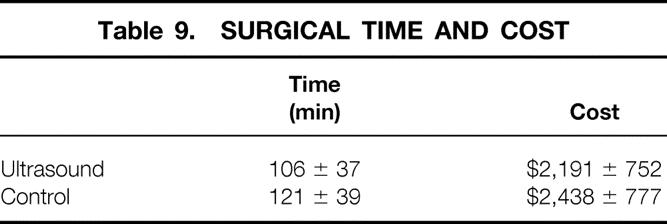
Satisfaction with the cosmetic aspect of surgery was equivalent between groups. Twenty-five of 27 patients in the IOUS group and 22 of 24 patients in the control group (Table 8) rated the cosmetic outcome 4 or 5 on a 5-point scale. We found no difference in the length of surgery or the cost generated from operating room expenses between the groups (Table 9). The average time for reexcision of margins for the eight patients who underwent this procedure (see Table 4) was 75 ± 26 minutes, and this generated an average cost of $1,788 ± 688 per case.
Table 8. COSMETIC EVALUATION
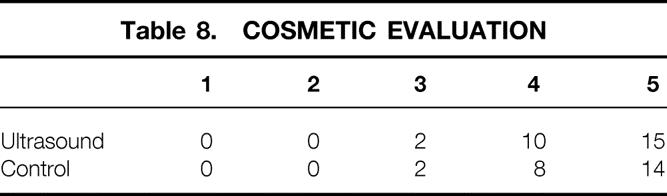
Table 9. SURGICAL TIME AND COST
DISCUSSION
Margin Status and Prognosis
The importance of margin status after excision of the primary tumor as a part of BCT has been a subject of debate, particularly in the recent literature. Numerous studies have evaluated the type of cancer, the margin status, and the amount of residual carcinoma in reexcision lumpectomy specimens. In an attempt to understand what impact these factors alone or in combination might have on overall local recurrence or survival, Gwin et al 4 evaluated 192 reexcision lumpectomy specimens and analyzed the relationship between clinical parameters and the characteristics of the excision of the residual tumor. In their analysis, 105 of the 192 patients (54.7%) had no residual tumor, 24% had minimal residual microscopic disease, 12% had extensive residual microscopic disease, and 9.4% had gross residual tumors. That study emphasized that when the initial resection margins were microscopically positive, a significant proportion of patients could be predicted to have nontrivial residual tumor. The results of that study predicted that almost 20% of patients with such reexcisions would have either gross or extensive microscopic residual disease. The authors strongly suggested that such patients would have less optimal survival and recurrence rates.
The significance of margin status in predicting the outcome of patients treated with BCT was reported by Solin et al 5 in their analysis of 697 consecutive women with clinical stage I or II invasive carcinoma of the breast who were treated with BCT and postoperative radiation. Their study evaluated four groups of patients: 250 patients with a negative margin (>2 mm), 57 patients with a microscopically positive margins, 37 patients with a close margin (<2 mm), and 346 patients with an unknown margin. This study used wider “boost fields” of radiation in patients with positive or close margins. Ultimately, however, there was no significant difference among the four groups’ 5-year actuarial survival or recurrence rates. This study concluded that selected patients with positive or close microscopic margins could be adequately treated with results comparable to those in margin-free patients.
The data in other studies are not so clear. Borger et al 6 evaluated 1,026 patients with clinical stage I and II breast cancer at the Netherlands Cancer Institute. These authors examined numerous factors in an attempt to demonstrate increased risks of local recurrence and showed that higher recurrence rates occurred in patients younger than 40 and in patients with tumor showing vascular invasion. The role of margin involvement was reported to be “uncertain.” This report did show, however, that patients with extensive ductal carcinoma in situ involved within the resected specimen had an increased local failure rate, raising the question of careful assessment not only of the margins but also of the extent of the intraductal opponent of the initial pathology.
DiBiase et al 7 evaluated the margin status in 453 patients treated with BCT. They evaluated the overall number of positive margins in an attempt to quantitate local recurrence. Rather than evaluating the inked margins of the excised tissue mass, they excised arcs of additional tissue by shaving the edges of the excised cavity and evaluating those as individual margins. In this fashion, they could identify the individual margins exactly and their relation to the original tumor (e.g., superior, medial, lateral, deep). They reported that local tumor control rates for patients with negative margins at 5 and 10 years were 94% and 87%, respectively. This compared favorably with patients with positive margins, for whom 86% 5-year and 69% 10-year control rates were reported. Breaking out the group with one positive margin and comparing it with the negative margin group showed no significant difference in local tumor control. Therefore, the group concluded that women with two or more positive margins had worse local tumor control than the negative margin group.
Gage et al 8 reached a different conclusion. They studied 343 women with extensive intraductal carcinoma and evaluated the inked margins of the excision specimen. Using a 1-mm distance as a negative margin, they found that the 5-year rate of ipsilateral breast recurrence in patients with negative margins was 2%, compared with 16% in patients with positive margins.
Assersohn et al 9 evaluated 184 patients, defining a positive margin as less than 1 mm from the excision. They reported that after the initial excision, 38% of the patients had microscopically involved margins. The local relapse rate after a follow-up of 5 years was only 1.9% and did not differ significantly from the uninvolved group. In addition, there was no difference in the distant relapse rate and survival between the positive and negative margin group.
The current literature continues to debate not only the long-term effects of margin status on survival but also what constitutes an adequate margin. Other recent reports have underscored the importance of obtaining as much information about margin status at the time of excision to address the ultimate question as to the impact of positive margins, near margins, or tumor-free margins on survival and local recurrence. 10–12 Although what constitutes an appropriate definition of a “positive” or a “negative” margin is unclear, it is very clear that analysis of the margins is important, possibly for survival and local recurrence and definitely in terms of patient and surgeon satisfaction with the overall results. At the very least, it is apparent that the best survival statistics clearly occur in the margin-negative or minimally involved groups. Therefore, it would seem logical to continue to evaluate postoperative specimen involvement with tumor as well as with preoperative or intraoperative methodologies that would ensure, at the very least, minimal involvement of the margin by the excised primary tumor in patients electing BCT.
Imaging and Assessment
Various imaging modalities have been used to locate and stage tumors before surgery and to locate and remove tumors and masses during surgery. Ultrasound has particularly lent itself to both preoperative and intraoperative use. During the past 10 years, ultrasound has been used in conjunction with mammography to evaluate the location and characteristics of breast lesions. The increase in the use of ultrasound as an adjunctive modality is directly related to the development of high-frequency linear-array transducers in conjunction with computer-enhanced image capabilities. 1,13–15 Several recent reports have evaluated the use of ultrasound in the evaluation of palpable breast lesions. Georgian-Smith et al 16 evaluated 293 palpable breast malignancies with an ultrasound scanner using a 5- to 10-mHz linear-array transducer. They could detect all 293 palpable malignant lesions. In addition, 18 of these lesions were occult mammographically. Tresserra et al 17 performed a retrospective review on 174 cases where breast tumor size was measured by sonography before surgery and compared after surgery with the pathology reports. They reported that the sonographic measurements of the tumor size correlated well with the pathologic specimen. This correlation was greater for lesions of 2 cm or less in greatest diameter. In addition, tumors with an extensive intraductal component were more difficult to evaluate using ultrasound than those without such intraductal involvement.
Initial reports are appearing on the use of IOUS to guide surgery and to confirm complete resection. 13,18 Rahusen et al 19 prospectively evaluated 19 patients with 20 mammographically nonpalpable lesions. They used a 10-mHz transducer during surgery to localize the tumor and plan the excision. They then compared their experience with 43 wire localization excisions performed during the same period. In the wire excision group, only 17 of 43 (40%) resections were deemed to have “adequate margins.” In the 20 excisions using ultrasound guidance, 7 (89%) had acceptable margins.
Harlow et al 2 also used IOUS to guide removal of nonpalpable breast carcinomas. Using a 7.5-mHz linear-array ultrasound probe, they removed 65 breast cancers in 62 patients. They reported an overall success rate in achieving pathologically negative margins at the first procedure of 97% (63/65).
Use of Imaging for Resection of Palpable Breast Tumor
In this study we addressed the use of IOUS in helping the surgeon obtain margins as clear as possible, realizing that the literature has not yet solved the problem of what criteria absolutely establish a clear margin and what the ultimate prognosis is relative to the degree of “clear” margins. It seems reasonable to speculate that efficient removal of breast cancer, removing as little normal tissue as possible while gaining satisfactory distance from malignant tissue (surgical accuracy), is an optimal goal and that the use of IOUS is a cost-effective technology that would be helpful in obtaining the greatest degree of surgical accuracy. As a primary evaluation, we designed the study randomizing the two groups of patients with palpable breast cancers specifically to evaluate the efficacy of IOUS. We found, as did the Rahusen and Harlow studies, that a higher percentage of negative margin samples were obtained using ultrasound guidance. To our knowledge, few if any studies have been able to quantitate the surgical accuracy of our study by showing that the use of IOUS allowed a smaller sample of tissue to be removed (104 cm3 vs. 114 cm3) with better margin status (7.6 mm vs. 4.8 mm to closest margin). When IOUS was used, there was a significant reduction in the incidence of pathologically positive margins (3.5% vs. 29%). It is difficult to determine from our data whether extensive ductal carcinoma in situ is associated with an increased rate of positive margins, as in Borger et al’s study. 6 We have found no other reports, however, that suggest that the use of IOUS appears optimal in the patient with dense breast parenchyma surrounding the lesion as determined by preoperative mammography. Good cosmetic results were equivalent in both groups. Looking at our cost per case and the cost of required reexcision in the case of unsatisfactory margins, it is easy to conclude from our data that if the use of IOUS increases the surgical accuracy, fewer reexcisions (or conversions to mastectomy) will be required and a significant cost savings will be realized.
Finally, we showed that IOUS does not appreciably slow the surgical procedure. Indeed, we expect that the reverse may become true because the accurate assessment of tumor margins reduces the surgeon’s need for additional resection of close margins after initial specimen resection. Further, IOUS provides an important aid for avoiding resection of larger volumes of normal breast parenchyma, thereby improving overall cosmesis. Most importantly, if future studies confirm our initial findings that ultrasound-guided surgery decreases the need for second surgeries, IOUS may result in less expensive surgery with improved patient satisfaction and decreased emotional turmoil. Just as ultrasound has become a standard tool of the breast surgeon’s office practice, we propose a role for ultrasound in the accurate intraoperative assessment of palpable breast tumors as well.
Discussion
Dr. William C. Wood (Atlanta, Georgia): I rise to thank the authors for sending me your very fine paper and giving me the opportunity of reading it, and also to congratulate you for a randomized trial of a new technique. It is terribly important that as things are introduced that they be addressed with randomized trials, and we all recognize in surgery that that is the exception rather than the rule, and so I very much appreciate that. Furthermore, you have demonstrated that you are taking smaller specimens with a higher percentage of clear margins, which is clearly the goal. A few questions.
First of all, this was entirely confined to ductal carcinoma. Do you have any data on invasive lobular cancers? Have you done any of those? Secondly, was this really a comparison of ultrasound or not, or was it a comparison of very careful mapping and incision planning or not?
You describe with the ultrasound carefully in two planes, mapping and marking, where you would make the incision and then repeating that examination to make sure you had placed it very well. Did you do the same with palpation? Did you carefully measure in two planes, make your incision marks, and then repeat your manual examination to give yourself the same advantages there? Thirdly, did you compare size and location by palpation and by ultrasound in the office preoperatively? And did you find a disparity in some patients between what you found by ultrasound and by palpation, and was it the group with the disparity that seemed to further benefit? Fourth, not having done this, I’m trying to picture how this helps. Now you used it to locate where you made the incision, then once you were performing the operation, you described using it in the wound to judge your margin. And then, finally, you described ultrasound of the specimen to make sure you have a margin all the way around. Did each part contribute equivalently, or was there a difference?
Now my comment: You tried for a 1-cm margin. Unless the core biopsy shows extensive intraductal component, I try for a 3-mm margin. For a 1.4-cm tumor, the difference in volume between those two margins is a 7-cm3 mass versus a 29-cm3 mass, four times the volume. Now, actually, you had about 100-cm3 mass. Now you may have women with extremely generous breasts that you admitted to this study, or they may have a little bit of a defect from where these were removed. I have limited myself to using ten sensors linked by a very small computer to try and judge that 3-mm margin. It would appear that this study and the one before it provide further evidence of my advancing cerebral arteriosclerosis because I had always put the hematoma in the breast after my biopsy, Dr. Klimberg, not before it. And the embracing of new technology as part of my operative approach, I must admit, I find that I am a bit resistant to.
So even though I may not like your results, I loved your study, and I congratulate you for it.
Thank you.
Dr. R. Phillip Burns (Chattanooga, Tennessee): President Aust, Secretary Townsend, members, and guests. I, too, rise to compliment Drs. Moore, Hanks, and group on a well-designed study which offers a new application of an old diagnostic modality, breast ultrasound, to the use of ultrasound in helping solve a problem common to all surgeons who perform breast cancer treatment—that of inadequate margin after partial mastectomy or lumpectomy which, when it occurs, leads to both increased costs to the health care system and to the patient and, especially, to psychological stress to the patient if reexcision is required.
The text of the paper well describes the prospective design and standardization of operative technique and evaluation used in the study, and I recommend it to the audience for your review. I do have a few questions and comments.
The entry criterion was a palpable lesion with histologic confirmation of cancer by needle biopsy. Who did the biopsy? In what setting? And, if someone other than a surgeon, why?
Were these biopsies done free-hand, with guides, or with stereotactic or ultrasound guidance?
Many of these tumors were T1 lesions. Were they initially detected during self-exam, physician exam, or were they initially discovered as a result of a mammogram or ultrasound and then identified on physical examination?
The long-term cosmetic evaluation in both groups was good, so I assume there was no significant reaction or untoward effect from the use of ultrasound gel material inside the breast operatively. Please comment.
It is especially interesting that in the ultrasound-assisted group, the total specimen size was smaller with more favorable margins, although this did not reach statistical significance. This difference largely occurred in the patient subgroups classified on mammogram as having dense breast tissue, a patient group in which we tend to overexcise volumes of normal tissue more often in an effort to achieve adequate margins. This particular point argues well for many of us to consider adding this modality to our operative technique. Do you have further comment?
You report equal operating room costs with or without ultrasound use, primarily based on operative time. Where is the cost center for the ultrasound unit?
I am sure if most of us are going to add this to our operative technique, there will be a startup cost. What figure can we quote to our OR committees or administrators?
I found your detailed evaluation of the plane of closest margin in the ultrasound study of the specimen interesting, and especially the fact that the anterior margin was not as frequently the side of close margin, as is often reported in other series. Was the ultrasound of particular utility in this dimension, and did the use of ultrasound lead to excision of more skin over the palpable lesions and, therefore, less inadequate margins in this anterior plane?
Now that you have done this study, have you extended this technique to assist resection of nonpalpable tissue diagnosis-confirmed breast cancers? And, if so, are you using it in conjunction with wire or multiple wire localization techniques?
Again, I wish to compliment you on a well-designed clinical study and a very well-written manuscript, especially the discussion of the dilemmas we all face in defining an adequate margin in breast-conserving surgery. I wish to thank the Association for the privilege of discussing this paper.
Dr. Grace S. Rozycki (Atlanta, Georgia): This is an outstanding paper, and I just want to ask a quick question. For the patient who underwent ultrasound evaluation and had a positive margin, would you advise that surgeons purchase or use a wide-band frequency transducer that would allow higher resolution and possibly minimize the negative result? Thank you.
Dr. V. Suzanne Klimberg (Little Rock, Arkansas): I just would like to say that this technique frees you from radiology and scheduling, particularly. And I think this is an important part of what you can do. You are freed up from that so you can go directly to the operating room.
The question I want to ask is, I can’t comprehend that this would not be less expensive, because it also frees you from the cost of radiology, which is not only the procedure itself, which far exceeds the cost that you charge intraoperatively for ultrasound, but also the reading that they do, which is another additional cost. Did you take those into account in your cost estimates?
Thank you.
Dr. Marcia Moore (Charlottesville, Virginia): President Aust, Secretary Townsend, members, and guests. I want to preface my remarks by thanking Dr. Burns and Dr. Wood for their kind review of the manuscript in advance and for their kind questions. I will try to take them in order.
Dr. Wood began by discussing infiltrating ductal cancers versus infiltrating lobular, as he noted that we had excluded infiltrating lobulars from our group. And the reason we did that is in response to our initial studies that we published from this group last year, showing that our positive margin rate is much higher in infiltrating lobular cancers. And we felt that we would have a more sensitive study if we could restrict ourselves initially to infiltrating ductal cancers. Indeed, the gist of most of my discussion today will be a plea to the membership to try to join with us to do additional studies of this sort. I think there are many that could come from this. I would love to see infiltrating lobular cancers included in this group.
Dr. Wood wanted to know which portion of the ultrasound procedure was the most helpful. It may indeed be that it helps us in the incision planning, and, as Dr. Burns pointed out, in assessing the anterior margin, although we did not find that the anterior margin was the problem most of the time we had expected we might.
I think that we do not have the data to tell you whether we are looking at the difference in size from palpable versus ultrasound, though, in general, the way the ultrasound helps us, and as Dr. Rozycki points out, could help us even more with the appropriate tools, is to help us further define the edges of the tumor.
The reason that I began this study actually came from my beginning to do injection procedures for sentinel node. I have spoken with many of the experts on sentinel node surgery, and almost none of them have the experience that I have, which is that when I inject 5 cc of isosulfane blue peritumorally, I reduce my own tactile feedback for the edge of the tumor and I furthermore stain the tissues with this brilliant cerulean blue, a lovely color that I just used to paint my dining room, but, nevertheless, what it does is reduce my ability to see the edge of the tumor and to feel the edge of the tumor. The ultrasound will help restore to you some of the tactile ability that I believe you use with the fluid that is injected around the tumor as part of your sentinel node procedure.
Dr. Wood has published several times on the benefits of having a 3-mm margin. I think a 3-mm margin is technically adequate. Unfortunately, I seem to need to aim for a 1-cm margin to even get a 5-mm margin. If I aimed for a 3-mm margin, I think I’d have positive margins all the time. But a 3-mm margin, I think, would be adequate if I only had a way to get it. It may be with Dr. Rozycki’s help and some additional ultrasound techniques we may ultimately be able to have a 3-mm margin.
I do want to encourage again any members who would be interested in a multicenter evaluation of this technique. I would encourage us to do it. I think that it would provide some additional information about surgeons being able to use ultrasound efficiently in the operating room.
Dr. Klimberg asked several questions that have to do with cost comparison. I did not add any cost to the patient for ultrasound because this was being done as part of a study, and we did not think it was ethical to bill the patient for that portion of our procedure. Because these are palpable lesions, we were not having radiologists involved in the control arm of the study, so there was not an offsetting radiologic cost.
Thank you for your attention.
Footnotes
Presented at the 112th Meeting of the Southern Surgical Association December 4-6, Palm Beach, Florida.
Correspondence: Marcia Moore, MD, Martha Jefferson Physician Hospital Organization, 310 Old Ivy Way, Charlottesville, VA 22903.
E-mail: mmm7k@virginia.edu
Accepted for publication December 2000.
References
- 1.Makuuchi M, Torzilli G, Machi J. History of intra-operative ultrasound. Ultrasound Med Biol 1998; 24: 1229–1242. [DOI] [PubMed] [Google Scholar]
- 2.Harlow SP, Krag DN, Ames SE, et al. Intraoperative ultrasound localization to guide surgical excision of non-palpable breast carcinoma. J Am Coll Surg 1999; 189: 241–246. [DOI] [PubMed] [Google Scholar]
- 3.Moore M, Borossa BA, Imbrie JZ, et al. The association of infiltrating lobular carcinoma with positive surgical margins after breast conservation therapy. Ann Surg 2000; 231: 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gwin JL, Eisenberg BL, Hoffman JP, et al. Incidence of gross and microscopic carcinoma in specimens from patients with breast cancer after re-excision lumpectomy. Ann Surg 1993; 218: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solin LJ, Fowble BL, Schultz MA, et al. The significance of the pathology margins of the tumor excision on the outcome of patients treated with definitive irradiation for early state breast cancer. Int J Radiation Oncol Biol Phys 1991; 21: 279–287. [DOI] [PubMed] [Google Scholar]
- 6.Borger J, Kemperman H, Hart A, et al. Risk factors in breast-conservation therapy. J Clin Oncol 1994; : 653–660. [DOI] [PubMed] [Google Scholar]
- 7.DiBiase SJ, Komarnicky LT, Schwartz GF, et al. The number of positive margins influences the outcome of women treated with breast preservation for early-stage breast carcinoma. Cancer 1998; 82: 2212–2220. [PubMed] [Google Scholar]
- 8.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and risk of recurrence in patients treated with breast-conserving therapy. Cancer 1996; 78: 1921–1928. [DOI] [PubMed] [Google Scholar]
- 9.Assersohn L, Powles TJ, Ashley S, et al. Local relapse in primary breast cancer patients with un-excised positive surgical margins after lumpectomy, radiotherapy and chemoendocrine therapy. Ann Oncol 1999; 10: 1451–1455. [DOI] [PubMed] [Google Scholar]
- 10.Luu H, Otis CN, Reed WP, et al. The unsatisfactory margin in breast cancer surgery. Am J Surg 1999; 179: 362–366. [DOI] [PubMed] [Google Scholar]
- 11.Page DL, Johnson JE. Controversies in the local management of invasive and non-invasive breast cancer. Cancer Lett 1995; 90: 91–96. [DOI] [PubMed] [Google Scholar]
- 12.Vicini FA, Goldstein NS, Kestin LL. Pathologic and technical considerations in the treatment of ductal carcinoma in situ of the breast with lumpectomy and radiation therapy. Ann Oncol 1999; 10: 883–890. [DOI] [PubMed] [Google Scholar]
- 13.Stargen ED, O’Neil TP. Breast ultrasound. Surg Clin North Am 1998; 78: 219–236. [DOI] [PubMed] [Google Scholar]
- 14.Newman PG, Rozycki GS. The history of ultrasound. Surg Clin North Am 1998; 78: 179–196. [DOI] [PubMed] [Google Scholar]
- 15.Case TD. Ultrasound physics and instrumentation. Surg Clin North Am 1998; 78: 197–218. [DOI] [PubMed] [Google Scholar]
- 16.Georgian-Smith D, Taylor KJW, Madjar H, et al. Sonography of palpable breast cancer. J Clin Ultrasound 2000; 28: 211–216. [DOI] [PubMed] [Google Scholar]
- 17.Tresserra F, Feu J, Grases PJ, et al. Assessment of breast cancer size: sonographic and pathologic correlation. J Clin Ultrasound 1999; 27: 485–491. [DOI] [PubMed] [Google Scholar]
- 18.Tamaki Y, Sato Y, Nakamoto M, et al. Intraoperative navigation for breast cancer surgery using 3D ultrasound images. Comp Aid Surg 1999; 4: 37–44. [DOI] [PubMed] [Google Scholar]
- 19.Rahusen FD, van Amerongen AHMT, van Diest PJ, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancers: a feasibility study looking at the accuracy of obtained margins. J Surg Oncol 1999; 72: 72–76. [DOI] [PubMed] [Google Scholar]