Abstract
Objective
To examine clinical outcomes in patients receiving neoadjuvant chemoradiation for locally advanced rectal adenocarcinoma.
Summary Background Data
Preoperative radiation therapy, either alone or in combination with 5-fluorouracil-based chemotherapy, has proven both safe and effective in the treatment of rectal cancer. However, data are lacking regarding which subgroups of patients benefit from the therapy in terms of decreased local recurrence and increased survival rates.
Methods
A retrospective chart review was performed on 141 consecutive patients who received neoadjuvant chemoradiation (5-fluorouracil ± cisplatin and 4,500–5,040 cGy) for biopsy-proven locally advanced adenocarcinoma of the rectum. Surgery was performed 4 to 8 weeks after completion of chemoradiation. Standard statistical methods were used to analyze recurrence and survival.
Results
Median follow-up was 27 months, and mean age was 59 years (range 28–81). Mean tumor distance from the anal verge was 6 cm (range 1–15). Of those staged before surgery with endorectal ultrasound or magnetic resonance imaging, 57% of stage II patients and 82% of stage III patients were downstaged. The chemotherapeutic regimens were well tolerated, and resections were performed on 140 patients. The percentage of sphincter-sparing procedures increased from 20% before 1996 to 76% after 1996. On pathologic analysis, 24% of specimens were T0. However, postoperative pathologic T stage had no effect on either recurrence or survival. Positive lymph node status predicted increased local recurrence and decreased survival.
Conclusions
Neoadjuvant chemoradiation is safe, effective, and well tolerated. Postoperative lymph node status is the only independent predictor of recurrence and survival.
Local recurrence after treatment for locally advanced rectal cancer remains a problem. Until the mid-1980s, the standard of care for treatment of these tumors was surgical excision with a 2-cm distal margin. With this approach, however, local recurrence rates for tumors that extended through the muscularis propria (T3) ranged from 20% to 45%. 1–5 Initial efforts to improve local control and survival rates focused on postoperative adjuvant therapies. Both the Gastrointestinal Tumor Study Group and the North Central Cancer Treatment Group trials showed that combination chemotherapy and radiation therapy could reduce local recurrence to 10% to 15% while improving both disease-free survival and overall survival rates compared with either modality alone or to no treatment. 6–9 Based on the results of these studies, postoperative adjuvant chemoradiation became the standard of care for stage II and stage III rectal adenocarcinoma.
Continued efforts to improve local control and to maximize sphincter preservation led many to consider preoperative chemoradiation because of its many theoretical advantages: easier resectability from tumor downstaging, less small bowel radiation toxicity, avoidance of radiation on an anastomosis, and improved tolerance of the regimen. To explore these theoretical considerations, our group began to use neoadjuvant chemoradiation in 1986. A pilot study published in 1995 showed that patients treated before surgery had better local control and overall survival rates compared with a group of concurrent matched controls. 10 The current study examines the next hundred consecutive patients treated since 1995. The purpose of this study was to examine clinical outcomes in patients receiving neoadjuvant chemoradiation for locally-advanced rectal adenocarcinoma.
METHODS
One hundred forty-one patients with biopsy-proven rectal cancer without evidence of extrapelvic spread were treated neoadjuvantly. All patients underwent abdominal and pelvic computed tomography scans and chest x-rays. Local tumor stage was determined using endorectal ultrasound or magnetic resonance imaging with endorectal probe beginning in 1995. Lymph nodes were considered ultrasonically positive if they were enlarged, round rather than oval, irregular in border, or hypoechoic.
Radiation therapy was delivered by photon radiation generated by a 6-mV or greater linear accelerator. Attempts were made to exclude the small bowel from the radiation fields. The superior field of the anterior-posterior (AP) fields was the top of the fifth lumbar vertebra; the inferior border was the inferior border of the ischial tuberosities or 2 cm inferior to the most inferior aspect of the tumor. The lateral borders of the AP fields were 1.5 cm lateral to the pelvic brim, with the superior and anterior borders identical to those of the AP fields. The anterior border of these fields was the posterior edge of the pubic symphysis. For the first 54 patients, treatment was administered five times per week with a daily fraction of 180 cGy. Twenty-five treatments were delivered with a total pelvic dose of 45 G. For the remaining 87 patients, a boost of 540 cGy was added for a total of 5,040 cGy.
Chemotherapy for the first 54 patients consisted of 5-fluorouracil and cisplatin and was begun at the beginning of the radiation treatments. The dose of 5-fluorouracil for these patients was 500 mg/m2 per day, administered as a rapid infusion on 5 consecutive days, followed by a half-hour infusion of cisplatin (20 mg/m2 per day). The same chemotherapy was repeated during the last week of radiotherapy. The remaining 87 patients underwent chemotherapy consisting of 5-fluorouracil alone. Of these, 77 received continuous infusion administration of 5-fluorouracil alone in one of three dose regimens: 800 to 1,000 mg/m2 for 5 days in weeks 1 and 5; 1,000 mg/m2 for 4 days in weeks 1 and 5; or 225 mg/m2 for all 35 days. An additional 10 patients received an experimental oral 5-fluorouracil formulation along with eniluracil, an inhibitor of dihydropyrimidine dehydrogenase, as part of a research protocol.
Surgical treatment of the tumors was performed 4 to 8 weeks after completion of the chemoradiation. Surgical procedures included abdominoperineal resection, low anterior resection, transanal excision, and exploratory laparotomy. Since 1996, abdominoperineal resection and low anterior resection have been performed using sharp dissection according to the principles of total mesorectal excision. Pathologic response on postoperative staging was standardized such that the deepest bowel layer with microscopically viable tumor cells determined the T stage. Postoperative complications were tracked and entered into a database.
Recurrence and survival were first analyzed using the Kaplan-Meier method. The Mantel-Cox log-rank test was used to compare curves, with P < .05 considered significant. These outcomes were also analyzed using multiple regression analysis. The computer program Statistica for Windows version 5 (Statsoft, Tulsa, OK) was used for all statistical calculations and curve generation.
RESULTS
One hundred forty-one patients were treated with neoadjuvant chemoradiation. Mean follow-up was 33 months (median 27). Men outnumbered women by almost two to one, and the mean age was 59 years (range 28–81). Mean distance from the anal verge as measured by rigid proctoscopy was 6 cm (range 1–15). Endoscopic ultrasound was performed on 63 patients. Of these, 23 had uT3N0 disease and 40 patients met ultrasonic criteria for node-positive disease. Of these latter 40, 4 were uT2N1, 4 were uT3N2, and 32 were uT3N1.
The chemoradiation regimens were administered as soon as possible after diagnosis. The most common hematologic toxicity was diarrhea (7%). Although the numbers were small, less than half as many patients required dose reduction in the later cohort (5-fluorouracil alone and 5,040 cGy) compared with the patients receiving 5-fluorouracil and cisplatin. In the combination treatment group, 17% (9/54) of patients required dose reduction versus 6% (5/87) in the second cohort (5-fluorouracil alone).
The surgical procedures performed are presented in Table 1. One patient refused to undergo surgery after a clinical complete response to the neoadjuvant treatment. During the 14 years of this study, as shown in Figure 1, the number of abdominoperineal resections decreased while the proportion of low anterior resections increased. The percentage of sphincter-sparing procedures increased from 20% before 1996 to 76% after 1996. Of the patients undergoing low anterior resection, 86% had anastomoses protected by a temporary diverting ileostomy. Most patients underwent reconstructed with either a colonic J-pouch or coloplasty. Postoperative complications occurred in 31 patients (22%) and included five wound infections, two dehiscences, three small bowel obstructions, four cases of prolonged ileus, one anastomotic stricture, one bladder injury, two ureterocutaneous fistulas, one ischemic ileostomy, one peristomal hernia, one case of ostomy prolapse, and one case of rectal prolapse. Of the 65 patients who underwent low anterior resection, six anastomotic leaks developed for a leak rate of 9%. Of the patients undergoing abdominoperineal resection, presacral or pelvic abscesses developed in two (3%).
Table 1. SURGICAL PROCEDURES PERFORMED
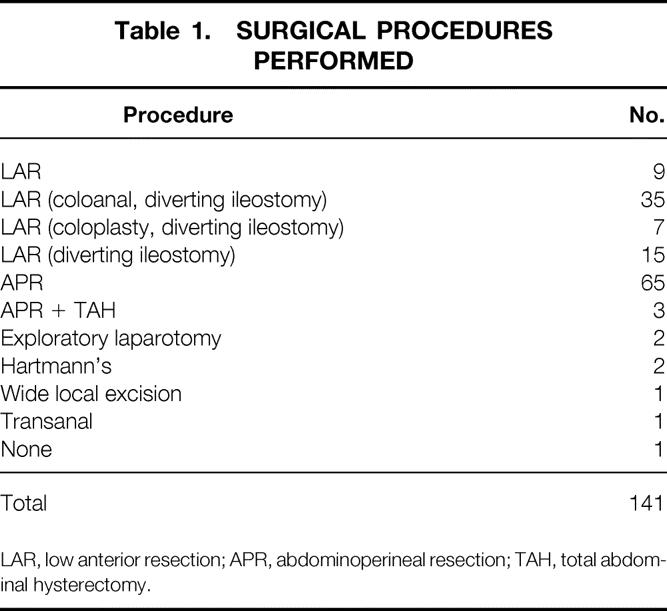
LAR, low anterior resection; APR, abdominoperineal resection; TAH, total abdominal hysterectomy.
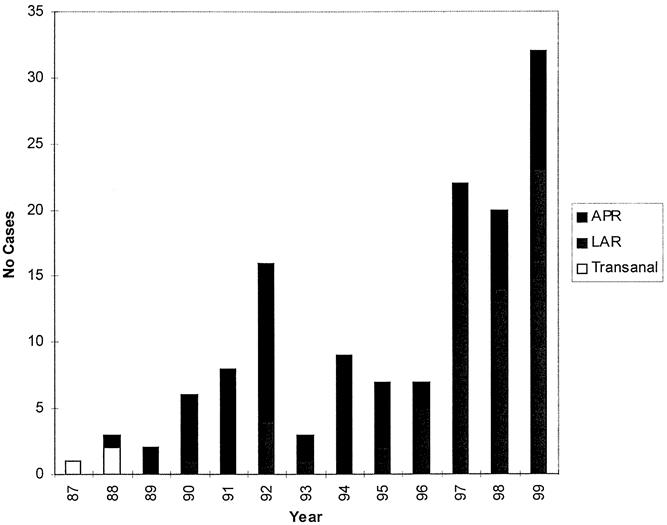
Figure 1. Surgical procedures by year. APR, abdominoperineal resection; LAR, low anterior resection.
The effects of chemoradiation on T and N stage are shown in Table 2. Sixty-three patients underwent preoperative endorectal ultrasound or magnetic resonance imaging staging. Examining T stage first, 40% of uT2, 55.6% of uT3, and 100% of uT4 patients were downstaged by the regimen. As Table 2 also shows, 75% of uN1 and 75% of uN2 patients were downstaged. Overall, considering both T and N stage, 57% of those without ultrasound evidence of nodal disease (stage II) were downstaged by the preoperative regimen and 11% had either pathologic positive nodes or distant metastases. Of these preoperative stage II patients, six (26%) had T0 specimens. Despite finding no viable tumor cells in the rectal wall, one of these six T0 patients had positive nodes on postoperative staging. Of those with positive nodes on preoperative ultrasound (stage III), 82% were downstaged by chemoradiation and 5% had either positive nodes or distant metastases. Of these preoperative stage III patients, eight (20%) had T0 specimens. Of these latter eight T0 patients, two had node-positive disease.
Table 2. PATHOLOGIC PRIMARY TUMOR DOWNSTAGING AND NODAL DOWNSTAGING OF PATIENTS WHO RECEIVED PREOPERATIVE EUS
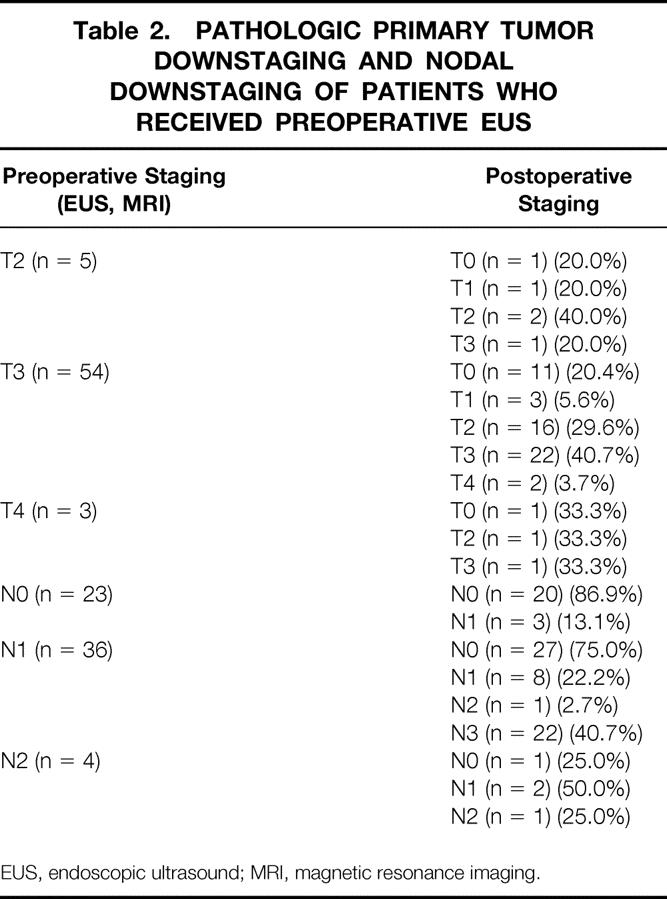
EUS, endoscopic ultrasound; MRI, magnetic resonance imaging.
Kaplan-Meier analysis was performed with local recurrence, disease-free survival, and overall survival as end points (curves not shown). Analysis of local recurrence as a function of time for all patients showed that seven patients had recurrence, for an actuarial 5-year local recurrence rate of 10%. Analysis of survival showed that the 5-year actuarial disease-free survival rate was 74% and the 5-year actuarial overall survival rate was 78%.
Kaplan-Meier and log-rank analyses were also performed to examine more closely the factors that may influence local recurrence and overall survival rates. The first factor analyzed was chemotherapeutic regimen. Early in our experience, 10 patients received 5-fluorouracil and cisplatin, whereas the successive patients received 5-fluorouracil only. There were no differences in local recurrence, disease-free survival, or overall survival rates between these two groups (data not shown).
Next, factors pertaining to surgery were analyzed. The vast majority of patients in the series underwent either abdominoperineal resection or low anterior resection. In analyzing local recurrence, disease-free survival, and overall survival rates in those treated by abdominoperineal resection versus low anterior resection, no significant differences were seen (data not shown). Because sharp mesorectal excision has been routinely performed at our institution since 1996, and because it has been shown to be instrumental in reducing local recurrence rates and in increasing survival rates, we examined whether sharp or blunt mesorectal dissection had a significant impact on patient outcome. Although sharp mesorectal excision led to a trend toward decreased local recurrence, this trend did not correlate with either the disease-free or overall survival rate.
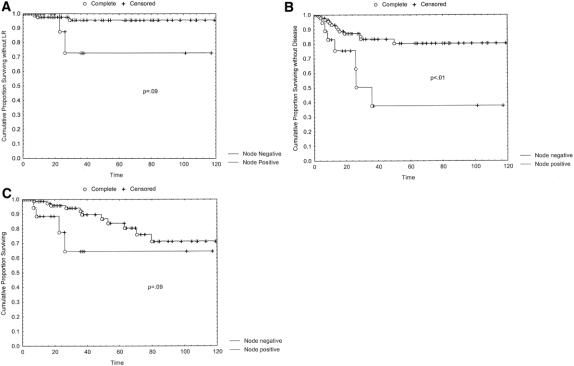
The influence of postoperative pathologic stage was also examined. Postoperative T stage was analyzed first. Tumor downstaging as a result of preoperative chemoradiation did not have a significant impact on the local recurrence, disease-free survival, or overall survival rate. In fact, even patients with T0 lesions after surgery showed no significant advantages in terms of local recurrence, disease-free survival, and overall survival compared with the entire cohort of patients with any residual T-stage disease (data not shown). Despite this, the presence of positive lymph nodes in the resected specimen portended a greater chance of local recurrence, with a probability value that trended toward significance (Fig. 2). In addition, as Figure 2 reveals, node-positive status had a strongly negative impact on the likelihood of long-term disease-free and overall survival.
Figure 2. (A) Local recurrence (LR), (B) disease-free survival, and (C) overall survival in patients with positive versus negative lymph nodes on postoperative pathologic analysis.
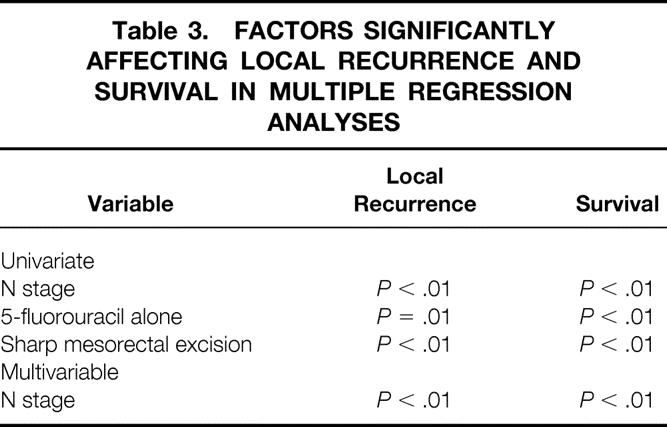
Finally, multiple regression analysis using both local recurrence and survival as dependent variables was performed Table 3. As Table 3 reveals, in examining both local recurrence and survival as an outcome in univariate analyses, N stage, use of 5-fluorouracil alone, and performance of a sharp mesorectal excision correlated significantly. However, when analyzing these three variables in a multivariate analysis, only N stage independently predicted overall survival.
Table 3. FACTORS SIGNIFICANTLY AFFECTING LOCAL RECURRENCE AND SURVIVAL IN MULTIPLE REGRESSION ANALYSES
DISCUSSION
Neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer is a widely practiced treatment before surgical excision. Although it was initially used to improve rates of sphincter preservation and to optimize patient tolerance, closer scrutiny may allow better individualization of treatment. For several years, preoperative radiotherapy in doses of 2,500 to 4,500 cGy has been given to patients with locally advanced rectal cancer. Although randomized studies were originally designed to reveal improvements in local recurrence, several studies also reported improved survival rates. 1,11–13 Addition of 5-fluorouracil-based chemotherapy to radiotherapy to radiosensitize the primary tumor and to eliminate systemic micrometastases has also improved rates of both recurrence-free 10,14–17 and overall survival 10,15–18 (Table 3). Considering our chemotherapeutic regimens, the use of 5-fluorouracil along with cisplatin conferred no advantage in local recurrence rates or survival and may have contributed to a higher complication rate compared with the use of 5-fluorouracil alone.
Our regimen appears to have achieved significant downstaging of rectal tumors. A T0 specimen rate (complete response at the primary tumor site) of 24% compares favorably with those in the literature for preoperative chemoradiation of 5% to 27%. 15,19–26 Indeed, some have suggested that preoperative chemoradiation can downstage such that sphincter preservation can be accomplished in some patients who would have required abdominoperineal resection. 20,21,27 Because of conflicting data on the ability to predict which patients are true pathologic complete responders 24,28 and because some T0 specimens contain N1 disease, our group believes that chemoradiation in most cases should not change the extent of the planned initial surgical procedure. The increased use of sphincter preservation in this study in recent years is not related to an attempt to minimize surgery in downstaged patients.
Given our success with downstaging, what is the importance of this downstaging for local recurrence and survival? Our results reveal that the postoperative pathologic appearance of the primary tumor affects neither recurrence nor survival, but residual tumor in lymph nodes predicts increased chances of recurrence. A recent study from the University of Florida showed similar data for N stage but revealed both decreased local recurrence and increased survival rates with T-stage reduction. 22 Addressing N stage first, overall survival in node-positive patients in response to a preoperative multimodality regimen trended toward statistical significance in Kaplan-Meier/log-rank analysis and achieved significance in multiple regression analysis. Longer follow-up is necessary to glean the importance of positive nodes. However, if overall survival worsens, two possible mechanistic explanations exist. First, pelvic nodal metastases may indicate hematogenous spread or may spread to distant sites themselves, ultimately leading to death. Alternatively, by rendering sensitive specimens node-negative, perhaps preoperative chemoradiation allows identification of patients whose tumors remain node-positive as having particularly aggressive tumors. In these patients, persistence of pelvic disease may be a marker for the presence of not just radioresistant but also fluorouracil-resistant cells beyond the pelvis. Such a finding would argue for the use of non-fluorouracil-based chemotherapeutic regimens before surgery.
In relation to the importance of posttreatment T stage, our data indicated that primary tumor response to neoadjuvant chemoradiation does not directly correlate with distant control and long-term survival. Although primary tumor response to neoadjuvant chemoradiation has been shown to have prognostic significance in esophageal cancer 29 and gastric cancer, 30 scant data exist regarding the importance of tumor downstaging at other primary sites. For breast cancer and pancreatic cancer, studies have shown nodal downstaging to be prognostic but have not analyzed primary tumor response. 31,32 One possible explanation for the disparity in the prognostic importance of T stage between this study and another 22 concerns the degree of downstaging. The complete response rate of 24% in the current series is nearly twice the 13% rate in the other series. Possible reasons for this difference include disparities in patient populations, treatment protocols, and pathologic practices. Of these possibilities, differences in treatment protocol (higher doses of fluorouracil and possibly higher radiation doses to more patients) seem most likely. Perhaps higher doses of fluorouracil allow better killing of micrometastatic disease, blunting the differences that would exist based on tumor responsiveness if lower doses are used.
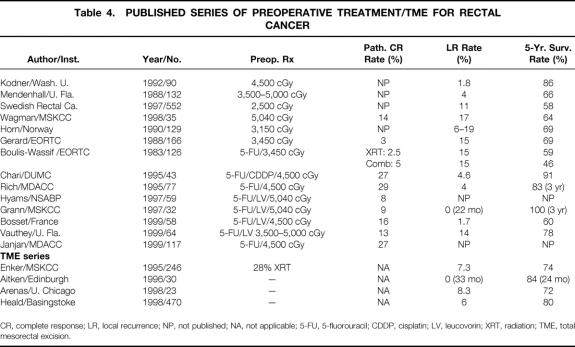
In the current series, the addition of sharp mesorectal excision since early 1996 has led to a trend toward decreased a local recurrence rate without significant increases in either disease-free or overall survival. However, other studies examining mesorectal excision alone have shown improvements in survival associated with a decreased local recurrence rate 33–35 (see Table 4). Much controversy exists as to whether chemoradiation adds additional disease control to rectal tumor resection performed with total sharp mesorectal excision. This issue is being addressed in the Dutch randomized trial of total sharp mesorectal excision alone versus total sharp mesorectal excision and radiation therapy. 36 In this country, as rectal resection with sharp mesorectal dissection becomes more widely performed, determining which tumors can be adequately treated with appropriate surgical technique alone and which require additional chemoradiation will be important. Perhaps sharp mesorectal excision reduces local recurrence so effectively that chemoradiation will add only a small degree of improvement. If this is the case, a much larger group must be studied to reveal a significant response to neoadjuvant chemoradiation.
Table 4. PUBLISHED SERIES OF PREOPERATIVE TREATMENT/TME FOR RECTAL CANCER
CR, complete response; LR, local recurrence; NP, not published; NA, not applicable; 5-FU, 5-fluorouracil; CDDP, cisplatin; LV, leucovorin; XRT, radiation; TME, total mesorectal excision.
Although longer follow-up is necessary to evaluate what sharp mesorectal excision may add in current neoadjuvant chemoradiation management strategies to treat locally advanced rectal tumors, several conclusions are possible from these patients. First, the presence of tumor in resected lymph nodes conferred much lower chances of recurrence-free and overall survival. Second, the T-stage response to neoadjuvant chemoradiation did not correlate with either freedom from recurrence or long-term survival. The fact that other series have shown otherwise signals the need for prospective, randomized trials involving neoadjuvant chemoradiation.
Future neoadjuvant trials should focus on obtaining tissue from primary tumors and enlarged lymph nodes before and after treatment. With modern molecular biology techniques, evaluation of gene expression and chemotherapeutic resistance markers can be performed. Correlation between resistance markers or other molecular markers and the propensity of cells to metastasize can be identified. The ability to stratify patients based on molecular markers instead of on biologic tumor response may prove to be a more accurate method of identifying patients for whom neoadjuvant chemotherapy will have most benefit.
Discussion
Dr. Kirby I. Bland (Birmingham, Alabama): President Aust, Secretary Townsend, fellows, and guests. I apologize for rising again; I was asked to discuss this in advance. I thank the authors for forwarding the manuscript well in advance, and I encourage you to look at this. There is a wealth of information in it that Doug could not present.
The authors have studied the clinical outcomes in 141 consecutive patients receiving preoperative neoadjuvant chemoradiation for locally advanced rectal adenocarcinoma. Like many groups nationally and internationally conducting such nonrandomized rectal trials, the chemotherapeutic regimen of choice has been 5-FU, which has shown therapeutic activity against adenocarcinoma and is also known to be a radiosensitizer. Initially, the authors treated 54 of the patients with 5-FU plus an infusion of cisplatin, as Doug has related, but they discontinued this combination for two reasons: presumably, because of its lack of enhancing antitumor activity and because of the associated toxicity. Because of the negative outcomes that he has reported here, however, it is encouraging to learn that 58% of patients without ultrasound evidence of nodal disease were downstaged by these preoperative regimens; 11% had either node-positive or distant disease in the final analysis.
Further, of the preoperative stage II patients, 21% had T0 specimens, implying that there was complete resolution of the index rectal tumor following chemoradiation. Of significance is the fact that for patients with positive nodes on preoperative ultrasound (clinical stage III), three quarters were downstaged following the technique and one fifth of this stage III cohort were converted to T0 specimens, of which one third of that total group had positive nodes. Thus, it is evident by this study, as well as previous studies reported before this Association, that preoperative chemoradiation has the salient benefit to impact downstaging and locoregional control.
In two previous studies from the University of Florida, we reported on before this group, the advantages of the addition of 5-FU and, in one case leucovorin, with preoperative radiation to enhance locoregional control and overall survival.
I have some questions and comments for the authors.
The authors have carefully analyzed the influence of postoperative pathological stage following preoperative chemoradiation. The significant frequency of tumor downstaging (76% in ultrasound-positive patients) did not impact local recurrence or survival. However, it is the presence of regional lymphatics that portends the highest frequency of local control and reduction in disease-free survival. Did the authors detect differences in overall or disease-free survival among the cohort of patients in whom chemoradiation downstaging caused their suspicious regional lymphatics (detected initially through endorectal ultrasound) to be converted to pathological disease-free specimens?
Further, does this observation suggest selective sensitivity to the chemoradiation regimen for this cohort in whom tumor response is evident?
Neoadjuvant chemotherapy–irradiation for locally advanced tumors was initially conceived to enhance locoregional control and sphincter preservation. The addition of cisplatin to your 5-FU radiation protocol appeared to have no salient benefit in the Duke series. What do you currently consider the state-of-the-art protocol, chemotherapy combined with radiation, in treating locally advanced tumors?
And my last point is a comment. We have tried in a number of studies nationally, and a more recent study which actually has been stopped was the NSABP trial in which we tried to randomize preop and postop radiation. That has been stopped after approximately 300 patients, and we are hoping that in the oncology trial groups of the American College of Surgeons that we can gain some impetus and encourage you to put patients on those trials that are going to be conducted. And Dr. Al Cohen, who is in the audience, is actually the organ site chair for that group. And I think this has to be answered, but the other part of it will be you will have to have a very homogenous group of patients to study to answer these questions.
I think it is a very important study. I congratulate the authors and thank you for the floor.
Dr. Jean-Nicolas Vauthey (Houston, Texas): I would like to thank Dr. Onaitis and Dr. Tyler for another excellent paper on neoadjuvant chemoradiation for locally advanced rectal cancer. The authors indicated effective local therapy with a low local recurrence rate and downstaging to lower pathological T categories in the majority of their patients. Unfortunately, the T downstaging was not associated with nodal negativity. Of importance, several patients with positive lymph nodes were present in the PT0 group, the group with complete tumor response. And this most likely affected the correlation between PT downstaging and survival.
This is in contrast with the experience at M.D. Anderson Cancer Center and also at the University of Florida, the data of which was presented to you 2 years ago at this meeting, showing a correlation between PT downstaging and survival. What makes the Duke experience different?
At the first two institutions, continuous infusion chemotherapy has been used preferentially because of the short half life of 5-FU. The treatment is given every day for 5 weeks during the entire course of radiation. In multivariate analysis presented here 2 years ago, continuous infusion chemotherapy was a significant factor for nodal downstaging in multivariate analysis. And continuous infusion chemotherapy is probably or perhaps the mechanism by which systemic micrometastatic disease is prevented or eradicated. I have two questions for the authors.
How many patients in your series received continuous infusion chemotherapy and what was the dose? Since nodal downstaging is associated with survival, did you notice any difference in nodal downstaging in association with the various chemotherapy regimens?
I’d like to thank the Association for the privilege of the floor.
Dr. Martin J. Heslin (Birmingham, Alabama): Dr. Aust, Dr. Townsend, thank you. I’d like to also congratulate Dr. Onaitis and Dr. Tyler on an excellent study. Basically I have two questions.
The recent long-term follow-up of the R02 trial from the NASBP suggests that there may not be as big a component for radiation therapy to reduce local recurrence, with the local recurrence rates of approximately 9% in the patients who receive radiation and 14% in those who did not. Perhaps from improvements or popularization of total mesorectal excision. Your study, as others with preop chemoradiation, has shown that sphincter preservation rates can approach 75%, even in those patients that preoperatively have only been recommended APR previously.
So my question would be, do you believe that it is the radiation therapy that is improving sphincter preservation rates and it is the surgical technique that is reducing local recurrence, or is there any way to separate those factors in your study?
The second question relates to survival advantage in those patients that have response versus none. In your study, the overall response rate was very high, suggesting that if you include CR and PR, more than half the patients had some response to radiation and chemotherapy. Similarly, you had a relatively low event rate, meaning that not many people died. So could the lack of survival advantage be due to the low number of events?
I’d like to thank the Association for the opportunity to discuss. Thank you.
Dr. Mark W. Onaitis (Durham, North Carolina): Thank you, President Aust and Secretary Townsend, for the opportunity to close today. I will answer these questions in order. I’d like to thank the discussants also for the questions.
Addressing Dr. Bland’s first question: Were there differences by N stage in disease-free survival or overall survival? We did look at those patients: patients who were preoperatively N0 and preoperatively N1 fared no differently in Kaplan-Meier analyses, either for local recurrence, disease-free recurrence, or overall survival. In addition, he asked if there was a cohort of patients who could be selected out who we could predict would do better with this therapy, which patients would respond preoperatively. We have shown that N stage is the only prognostic factor that seems to make a difference postoperatively. Perhaps trials that look at molecular markers of possibly ability to metabolize 5-FU or be radioresistant would be helpful in addressing preoperatively which patients are going to respond to the therapy.
As pertains which is the standard of care at our institution for neoadjuvant chemoradiation regimens, we have stopped using cisplatin, partially because it has not been an effective adjuvant therapy to 5-FU alone. Also, it seems to increase the complication rate, and we have gone to using 5,040 cGy of radiation in order to treat these patients pelvically. This has been our regimen and it seems to have worked, with adequate results in the last 87 patients in the series.
Turning to Dr. Vauthey’s questions. Our N-positive patients who are also T0, and there were 4 of them out of 141 in our series, none of them have as of yet recurred, as well as none of them have died. So none of them have entered into the analysis in a negative way. So that cannot explain the difference between T-stage response in our series.
As pertains to our continuous infusion therapy, we had 80 patients who got continuous infusion 5-FU. They got a dose of 225 mg/m2 per day, and when we broke down the analysis, and this really makes the number somewhat small, we saw no difference between types of 5-FU given and ultimate response to the therapy as well as local recurrence and overall survival. So whether they got bolus 5-FU, continuous infusion 5-FU, or an experimental oral formation that 20 of our patients got, none of them had any differences in survival.
As pertains to differences in nodal downstaging between groups, this was partially answered by Dr. Bland’s second question. I don’t think there is any way yet to identify preoperatively which patients will respond to preoperative chemoradiation and which patients will not. Perhaps further trials will help us in that area.
Pertaining to Dr. Heslin’s two questions. As far as radiation therapy or technique and the relative benefits of both on sphincter preservation, our feeling is that our improved rates of sphincter preservation in the latter group after 1996, where 76% had their sphincters preserved, is mainly due to operative technique improvements, being more comfortable with performing low anastomoses, and not from the radiation treatment itself, although previous to 1996 and 1995, when we did not use sharp mesorectal excision, we also had a very low local recurrence rate, suggesting that radiation does have some effect on the amount of tumor burden left on the pelvis.
As far as survival advantage and whether we have a low number of events, I think that at least for local recurrence, in several large series now, it has been shown that total mesorectal excision alone has a single-digit percentage of local recurrence rate. I think that we are probably underpowered to show much significant difference between total mesorectal excision and chemoradiation, although we didn’t look at that in this study. That’s probably a study that is going to require large numbers to show a very small difference.
As far as survival advantage, I think time will tell. We will have to follow more patients for a longer period of time to show ultimate survival advantage from this therapy.
Again, I’d like to thank the group for the opportunity to present this paper.
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Dr. Douglas S. Tyler, Department of Surgery, Duke University Medical Center, DUMC Box 3118, Durham, NC 27710.
E-mail: tyler002@acpub.duke.edu
Accepted for publication December 2000.
References
- 1.Pahlman L, Swedish Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336: 980–987. [DOI] [PubMed] [Google Scholar]
- 2.Pilipshen SJ, Heilwell M, Quan SH, et al. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984; 53: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 3.Wanebo HJ, Kones RJ, Veseridis MP, et al. Pelvic resection of recurrent rectal cancer. Ann Surg 1994; 220: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingleton WW, Prosnitz LR. Adjuvant therapy for colorectal cancer. Curr Probl Cancer 1985; 9: 1–34. [DOI] [PubMed] [Google Scholar]
- 5.Harnsberger JR, Vernava VM, Longo WE. Radical abdominopelvic lymphadenectomy: historic perspective and current role in the surgical management of rectal cancer. Dis Colon Rectum 1994; 37: 73–87. [DOI] [PubMed] [Google Scholar]
- 6.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically-treated rectal carcinoma. N Engl J Med 1985; 312: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 7.Krook JE, Moertel CG, Mayer RJ, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–715. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell MJ, Martenson JA, Weiand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994; 331: 502–507. [DOI] [PubMed] [Google Scholar]
- 9.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990; 264: 1444–1450. [PubMed] [Google Scholar]
- 10.Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995; 221: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodner IJ, Shemesh EI, Fry RD, et al. Preoperative irradiation for rectal cancer. Ann Surg 1988; 209: 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh PJ, James RD, Schofield PF. Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma: results of a prospective, randomized trial. Dis Colon Rectum 1994; 37: 1205–1214. [DOI] [PubMed] [Google Scholar]
- 13.Cedermark B, Stockholm Colorectal Cancer Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol 1996; 3: 423–430. [DOI] [PubMed] [Google Scholar]
- 14.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer. Ann Surg 1999; 230: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stryker SJ, Kiel KD, Rademaker A, et al. Preoperative chemoradiation for stages II and III rectal carcinoma. Arch Surg 1996; 131: 514–519. [DOI] [PubMed] [Google Scholar]
- 16.Kaminsky-Forrett MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operative T3-T4 rectal cancer. Int J Radiat Oncol Biol Physics 1998; 42: 935–941. [DOI] [PubMed] [Google Scholar]
- 17.Rich TA, Skibber JM, Ajani JA, et al. Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Physics 1995; 32: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 18.Pucciarelli S, Friso ML, Toppan P, et al. Preoperative combined radiotherapy and chemotherapy for middle and lower rectal cancer: preliminary results. Ann Surg Oncol 2000; 7: 38–44. [DOI] [PubMed] [Google Scholar]
- 19.Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Physics 1998; 42: 51–57. [DOI] [PubMed] [Google Scholar]
- 20.Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum. Dis Colon Rectum 1997; 40: 131–139. [DOI] [PubMed] [Google Scholar]
- 21.Grann A, Minsky BD, Cohen AM, et al. Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 1997; 40: 515–522. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Marsh RW, Zlotecki RA, et al. Recent advances in the treatment and outcome of locally advanced rectal cancer. Ann Surg 1999; 229: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke SJ, Percapio BA, Knight DC, et al. Combined preoperative radiation and mitomycin/5-fluorouracil treatment for locally advanced rectal adenocarcinoma. J Am Coll Surg 1994; 187: 164–170. [DOI] [PubMed] [Google Scholar]
- 24.Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M.D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Physics 1999; 44: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 25.Boulis-Wassif S, Gerard A, Loygue J, et al. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone or in combination with 5-fluorouracil, followed by radical surgery. Cancer 1984; 53: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 26.Bosset JF, Magnin V, Maingon P, et al. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Physics 2000; 46: 323–327. [DOI] [PubMed] [Google Scholar]
- 27.Valentini V, Coco C, Cellini N, et al. Preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation. Int J Radiat Oncol Biol Physics 1998; 40: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 28.Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor density (RTCD): prognostic implications. Int J Radiat Oncol Biol Physics 1997; 37: 619–627. [DOI] [PubMed] [Google Scholar]
- 29.Orringer MB, Forastiere AA, Perez-Tamayo C, et al. Chemotherapy and radiation therapy before transhiatal esophagectomy for esophageal carcinoma. Ann Thorac Surg 1990; 49: 348–354. [DOI] [PubMed] [Google Scholar]
- 30.Lowy AM, Mansfield PF, Leach SD, et al. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 1999; 229: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meric F, Mirza NQ, Buzdar AU, et al. Prognostic implications of pathological lymph node status after preoperative chemotherapy for operable T3N0M0 breast cancer. Ann Surg Oncol 2000; 7: 435–440. [DOI] [PubMed] [Google Scholar]
- 32.Bold RJ, Hess KR, Pearson AS, et al. Prognostic factors in resectable pancreatic cancer: p53 and Bcl-2. J Gastrointest Surg 1999; 3: 263–77. [DOI] [PubMed] [Google Scholar]
- 33.Aitken RJ. Mesorectal excision for rectal cancer. Br J Surg 1996; 83: 214–216. [DOI] [PubMed] [Google Scholar]
- 34.Heald RJ, Moran BJ, Ryall RDH, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision. Arch Surg 1998; 133: 894–899. [DOI] [PubMed] [Google Scholar]
- 35.MacFarlane JK, Ryall RDH, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341: 457–460. [DOI] [PubMed] [Google Scholar]
- 36.Kapiteijn E, Klein Kranenbarg E, Steup WH, et al. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Eur J Surg 1999; 165: 410–420. [DOI] [PubMed] [Google Scholar]