Abstract
Objective
To measure coexpression of matrix metalloproteinase (MMP)-2, MMP-7, and MMP-9 genes by real-time quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) in benign and malignant phases of colorectal carcinogenesis.
Summary Background Data
Matrix metalloproteinases degrade and remodel the extracellular matrix and have been implicated in facilitating carcinoma cells to invade and metastasize. MMP-2, MMP-7, and MMP-9 have been shown to be overexpressed in various carcinomas; however, simultaneous examination of these enzymes in human normal mucosa, adenoma, and carcinoma has not been performed to date.
Methods
Between January 1, 1998, and June 15, 2000, 40 patients underwent colectomy and harvest and snap-freezing of normal mucosa, adenoma, and carcinoma. Five patients had adenoma and carcinoma in the same specimen; 35 had either adenoma (n = 6) or carcinoma (n = 29). Taqman qRT-PCR methodology was used to measure MMP gene copy number and normalized to β-actin RNA expression.
Results
The mean age was 62 ± 4 years, with 22 men and 18 women. One fifth of the adenomas exhibited severe dysplasia. MMP-7 gene expression was significantly increased in adenomas (43 times normal mucosa) but did not increase further in carcinomas (50 times normal mucosa). MMP-2 and MMP-9 were not different in adenomas (1.8 and 1.4 times normal mucosa, respectively) but were elevated in carcinomas (2.2 and 1.8 times normal mucosa, respectively). There was no correlation between size or dysplasia in adenomas or AJCC stage in carcinomas and MMP gene expression.
Conclusions
Overexpression of MMP-7 is an early event in the adenoma-to-carcinoma pathway, and expression does not appear to increase further in carcinomas. MMP-2 and MMP-9 appear to be primarily overexpressed in carcinomas. This may be one mechanism by which adenoma cells gain the ability to invade and carcinoma cells to metastasize.
Colorectal cancer is a major health concern, with approximately 140,000 new cases and 56,000 cancer deaths each year. There are clear genetic syndromes, such as familial adenomatous polyposis and hereditary nonpolyposis colon cancer, that increase the risk of colon cancer, primarily by the early onset and increased number of adenomatous polyps. 1 These syndromes are estimated to account for approximately 15% of all colon cancers and therefore make up the minority. Outside of known genetic predisposition, the largest risk for the development of colon cancer is in the patient with sporadic adenomatous polyps. 2 These adenomatous polyps and subsequent carcinoma appear to develop with sequential genetic alterations. 3 Multiple genes including p53, deleted in colon cancer, 2 and more recently alterations in matrix metalloproteinase (MMP) 4 have been associated with polyp development. The interrelationships between these alterations, polyp development, and progression to carcinoma are not well understood.
MMPs generally function to degrade proteoglycans and matrix glycoproteins. This process of remodeling of the extracellular matrix is an integral part of normal tissue growth and differentiation. However, unregulated degradation of the extracellular matrix in the process of carcinogenesis may lead to an advantage for the cancer cell. Loss of basement membrane integrity may correlate with an increased probability of distant metastasis and poor prognosis. 5 Therefore, overexpression of MMPs may be one part of the multistep process by which the neoplastic cell can proliferate and metastasize. To date, three MMPs have been most associated with colorectal adenomas and carcinoma. MMP-2 (gelatinase A) is associated with the degradation of type IV collagen. Overexpression has been reported in gastric, pancreatic, and colorectal cancer. 6,7 MMP-7 (matrilysin) appears to be expressed by the neoplastic cells and may function in the early phases of neoplastic growth. This was shown in colorectal adenomas compared with normal mucosa 4 and in mouse models of intestinal neoplasia. 8 Recent data have suggested that increased levels of MMP-9 (gelatinase B) RNA in colorectal cancer compared with normal mucosa were associated with significantly shorter disease-free and overall survival. 9 The correlation of these degradative forces in colorectal mucosa, adenomas, and carcinomas is not well understood in colorectal neoplasia.
The goal of this work is the simultaneous quantitation of mRNA expression for MMP-2, MMP-7, and MMP-9 in normal colorectal mucosa, adenomatous polyps, and invasive colorectal carcinoma from human tissues. This will provide useful data regarding patterns of expression for MMPs as normal mucosa transforms into adenomas and subsequently to carcinomas.
METHODS
Subjects
Between January 1, 1998, and June 15, 2000, 40 patients underwent colectomy and harvest and snap-freezing of normal mucosa, adenoma, and carcinoma. Five patients had adenoma and carcinoma in the same specimen; 35 had either adenoma (n = 6) or carcinoma (n = 29). One fifth of the adenomas showed severe dysplasia. There were 18 women and 22 men; the mean age was 62 ± 4 years. There was only one case each of pure tubular or pure villous adenoma, precluding meaningful analysis by this variable.
Tissue Processing
Normal colorectal mucosa, adenoma, and carcinoma tissue specimens were obtained by surgical excision (through an institutionally approved protocol) and inspected by a surgical pathology associate. The portions of the specimens to be used for quantitation of MMP mRNA were cut into approximately 50-mg fragments, snap-frozen in liquid nitrogen within minutes of removal from the patient, and stored at −80°C. On the day of sample analysis, the frozen tissue samples were removed from the −80°C freezer for processing. There was no attempt to microdissect the adenomatous specimens based on the presence or absence of dysplasia. If the polyp had any high-grade dysplasia on histologic evaluation, it was classified as such, recognizing that we could not be sure that the tissue processed for gene expression was the same exact tissue.
Isolation and Purification of Total RNA
Total RNA was initially isolated from tissue samples using TRIzol (Life Technologies, Gaithersburg, MD) per the manufacturer’s instructions. The total RNA pellet was dissolved in 100 μL diethyl pyrocarbonate (DEPC)-treated water and quantitated at 260 nm. A portion of the total RNA sample (75 μg) was further purified using RNeasy columns (Qiagen, Santa Clarita, CA). Briefly, samples were mixed with ethanol, loaded onto silica spin columns, and washed twice before being eluted with 50 μL DEPC-treated water.
Real-Time Quantitative Polymerase Chain Reaction
The system used involves a fluorogenic oligonucleotide probe. The fluorescence emitted is directly proportional to the number of template molecules in the reaction mixture. 10,11 The ABI PRISM 7700 Sequence Detector (Perkins-Elmer, Foster City, CA) is a thermal cycler designed to monitor multiple fluorescent signals in a 96-well format. Samples are amplified in optical polymerase chain reaction (PCR) tubes, eliminating the need for manipulation during the cycling or data collection steps. Using β-actin as an internal control, quantitation of the amount of target (copy number/pg β-actin RNA) in unknown samples is accomplished using the standard curve method. 12 This quantitative method was validated in our laboratory in a previous publication using Northern blot analyses 13 and subsequently for MMP-7 (data not shown).
Statistics
Descriptive statistics (mean, median, standard deviation) were calculated for baseline MMP values by tissue type (normal mucosa, adenoma, carcinoma). The Wilcoxon signed-ranks test was used to calculate differences in values between the tissue types to account for the paired nature of the data. Tests of association were calculated using the Spearman correlation coefficient. All data are presented as mean ± standard error of the mean. Significance was defined as P < .05.
RESULTS
Table 1 shows the clinicopathologic data associated with the patients studied. There was no correlation between severe dysplasia in adenomas and AJCC stage in carcinomas with gene expression in MMP-2, MMP-7, or MMP-9. Similarly, the size of either adenomas or carcinomas had no correlation with MMP expression.
Table 1. CLINICOPATHOLOGIC FACTORS
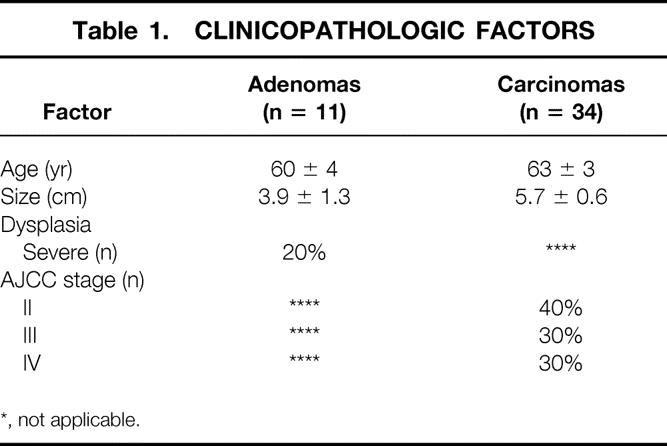
*, not applicable.
Table 2 reports the mean copy number of expressed genes per nanogram of RNA β-actin. MMP-7 was significantly increased in both adenomas and carcinomas, whereas MMP-2 and MMP-9 were significantly overexpressed only in carcinomas compared with normal mucosa. Figure 1 shows the relative expression of MMP-7: it was 43 and 50 times overexpressed in adenomas and carcinomas, respectively. Figure 2 shows the 1.8- and 2.2-times increase in expression of MMP-2 in adenomas and carcinomas, respectively. MMP-9 had a similar pattern of expression as MMP-2, with 1.4 and 1.8 times normal mucosa in adenomas and carcinomas, respectively (Fig. 3).
Table 2. MMP EXPRESSION BY TISSUE TYPE
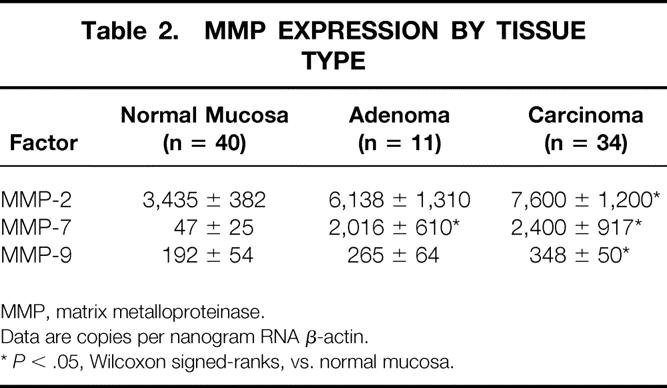
MMP, matrix metalloproteinase.
Data are copies per nanogram RNA β-actin.
*P < .05, Wilcoxon signed-ranks, vs. normal mucosa.
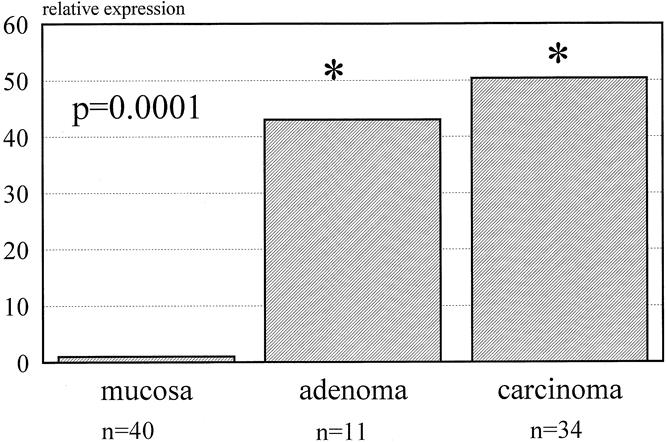
Figure 1. Expression of matrix metalloproteinase 7 for adenomas and carcinomas (relative to normal mucosa).
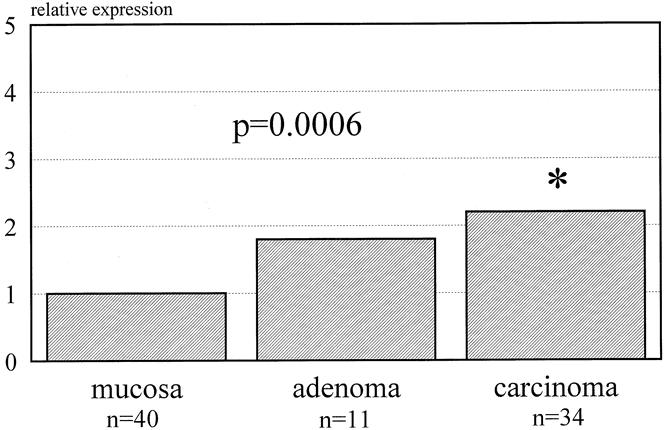
Figure 2. Expression of matrix metalloproteinase 2 for adenomas and carcinomas (relative to normal mucosa).
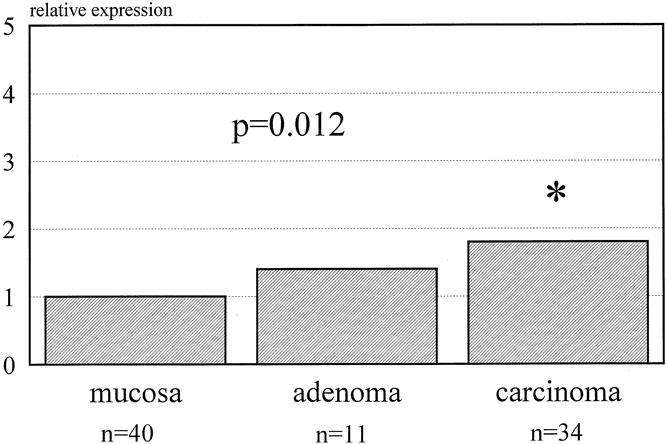
Figure 3. Expression of matrix metalloproteinase 9 for adenomas and carcinomas (relative to normal mucosa).
Table 3 shows the tests of association between MMPs in adenomas and carcinomas. There was a significant direct relationship between MMP-7 and MMP-9 in adenomas. In colorectal carcinomas, there was a direct relationship between MMP-7 and MMP-2 and a significant direct relationship between MMP-2 and MMP-9. These tests did not measure coregulation or coactivation but simply suggested that each was elevated in the same direction in the same tissues.
Table 3. TESTS OF ASSOCIATIONS BETWEEN MMPS
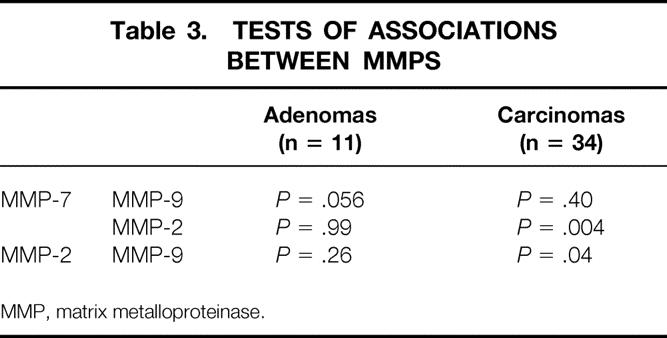
MMP, matrix metalloproteinase.
DISCUSSION
These data show the ability to measure simultaneously expression of multiple genes in human colorectal adenomas and carcinomas. Specifically, MMP-7 overexpression is an early event in the carcinogenic cascade as normal mucosa progresses to adenoma. As adenomas acquire the ability to become invasive adenocarcinomas, these data show that MMP-7 remains elevated and both MMP-2 and MMP-9 are overexpressed. This may allow for tumor cells to invade or for new blood vessel growth to occur. Expression of MMP-2 and 9 appears to be associated in invasive carcinomas.
Epithelial tumors are composed of tumor cells and surrounding stroma. The site of MMP overexpression/activation in a tumor seems to be different depending on the individual MMP. Biologically, the location of MMP overexpression may be an indication of a process intrinsic to the cancer cell or normal surrounding stromal cells being induced to express or secrete MMPs.
Newell et al 4 have shown that MMP-7 is primarily expressed on the tumor cell surface and therefore is likely a direct effect of disordered signaling pathways intrinsic to the cancer cell. This early change may allow abnormal cells to overcome normal cell–cell growth inhibition. Similarly, this may be the first step in the local proteolysis of the basement membrane to allow invasion. In vitro data have shown that MMP-7 expression is related to the invasiveness of the primary tumor cells. 14 The results of this study showed that MMP-7 levels in the tumor cells varied directly with the tumorigenicity. MMP-7 has been previously shown to be focally overexpressed in approximately 50% of benign adenomas 4 in one study and overexpressed in the more dysplastic and invasive portions of tumors by immunostaining in a separate study. 15 Others have shown that in patients with familial adenomatous polyposis, MMP-7 is constitutively overexpressed in all polyps regardless of size or dysplasia, whereas in sporadic adenomatous polyps, MMP-7 expression correlated with both size and dysplasia. 16 Overexpression of MMP-7 in adenocarcinomas is not limited to colorectal cancer. Previous work has shown that MMP-7 is overexpressed in 88% and 36% of invasive pancreatic 17 and breast 18 carcinomas, respectively. Interestingly, MMP-7 was overexpressed in 100% of in situ breast cancers studied in the latter publication, also suggesting an early role in the carcinogenic cascade. The results of the current study confirm that MMP-7 is an early event with significant overexpression in human adenomatous polyps, although there was no correlation with size or dysplasia. Invasive adenocarcinomas showed no correlation of MMP-7 expression with increasing AJCC stage in our study (data not shown); however, long-term follow-up with correlation to recurrence and survival remains to be completed.
MMP-2 and MMP-9 have been shown to be overexpressed by the stroma surrounding the tumor, 19 which may be a reaction to MMP-7 or other mediators known to induce MMP expression but not measured in the current study. This process of recruiting normal stromal cells surrounding tumor cells to produce MMPs seems to be an important mechanism by which proteolysis and subsequent invasion may occur. Swallow et al, 20 in an elegant in vitro study, evaluated colorectal cancer cell lines with and without metastatic potential for the ability to induce monocytes to produce MMP-2 and MMP-9. Each of the above cell lines was cocultured with monocytes but separated by a membrane, with MMP-2 and MMP-9 activity measured. Neither MMP was produced by the colorectal cancer cells. This study showed that colorectal cancer cell lines with metastatic potential had the ability to induce MMP-2 and MMP-9 activity in the stromal monocytes. For these particular MMPs, these in vitro data support the notion that cancer cells induce stromal cells to degrade the extracellular matrix and basement membrane through a paracrine-type effect. Poulsom et al 7 found that MMP-2 was also more heavily expressed in the stromal cells rather than tumor cells in an immunohistochemical study of colorectal adenomas and carcinomas. They reported 2 of 3 adenomas and 10 of 12 carcinomas had increased levels of MMP-2. Others reported that MMP-2 and MMP-9 were more expressed in the stroma surrounding squamous cell carcinomas of the skin versus nonmetastatic basal cell carcinomas. 21 These studies support our data showing an increased expression of MMP-2 and MMP-9 in colorectal carcinomas, although our data did not examine the actual cells from which the MMP mRNA was being extracted or localized. There is a possibility that because of insufficient numbers of adenomas, we could not statistically show an increase in adenomas compared with normal mucosa. With more numbers, we should be able to resolve this issue. The current study found no relationship between AJCC stage and MMP-2 or MMP-9 (data not shown).
One additional mechanism by which MMPs are proposed to increase the local invasiveness is to facilitate angiogenesis. Theoretically, local degradation of the basement membrane must occur for endothelial and tumor cells to invade normal tissues. Two recent studies support the role of MMPs in this process. Itoh et al 22 evaluated the invasive potential of cancer cells in MMP-2-deficient mice. They showed that tumors implanted in MMP-2-deficient mice had decreased invasive properties, suggesting that MMP-2 is important for invasion and that it is the normal stromal cells that are induced to facilitate invasion by upregulation and activation of MMP-2. This observation is supported by a study evaluating the effect of endostatin on tumor invasion. 23 Endostatin significantly reduced the invasive properties of tumor and normal endothelial cells, primarily by decreased activation and expression of MMP-2. Taken together, MMP-2 and potentially other MMPs appear to have a role in angiogenesis, and this may be blocked by angiogenesis inhibitors.
Correlation of MMP expression with patient survival has been studied in several tumor types. Zeng et al 9 evaluated the ratio of MMP-9 expression by Northern blot analysis in normal mucosa and carcinomas and correlated this ratio to recurrence and survival. An increased ratio was found to be significantly related to Dukes stage and the presence of distant metastases at the time of presentation and was an independent prognostic factor associated with a decreased disease-free survival. Sier et al 24 measured MMP-2 and MMP-9 activity in 50 patients with gastric cancer and found that the activity of both was increased in tumor tissue compared with normal mucosa. They found no correlation with MMP activity and TNM staging, but increased MMP activity was an independent predictor of overall survival.
Successful use of MMP inhibitors in the laboratory has been documented, with several clinical trials assessing efficacy in humans underway. Brand et al 25 evaluated the role of adenoviral delivery of tissue inhibitor of MMP (TIMP-2) for inhibiting metastatic colorectal carcinoma to the liver. They used a highly metastatic cell line (LS174T) that was shown to secrete MMP-2 primarily. Transfection of TIMP-2 into the liver before inoculation of tumor or after tumor was established decreased tumor burden by 95% and 77%, respectively. Reductions in proliferation and increases in apoptosis were also seen. Others have used antisense oligonucleotides to MMP-7 in a mouse model of colorectal metastases using splenic injection; they showed that treatment produced a 70% reduction in the number of metastases to the liver but no change in size in the ones that ultimately grew. 26 A more clinically relevant model assessed the role of a broad-spectrum MMP inhibitor (BB-94) in a orthotopic model of pancreatic carcinoma. 27 Tumor cells were injected into the pancreas at laparotomy and the animals were randomized to control, MMP inhibitor, gemcitabine, or MMP inhibitor plus gemcitabine. All treatment arms reduced the production of MMP-2 in the tumor, but the most significant reductions were seen in the group with the combination of MMP inhibitor and gemcitabine. The results of several clinical trials evaluating MMP inhibitors remain to be reported. It is likely that more specific inhibitors will have to be developed to see significant improvements in human cancer trials.
In conclusion, MMP-7 appears to be an early event in the adenoma-to-carcinoma pathway, with subsequent elevation in MMP-2 and MMP-9 in colorectal carcinomas (Fig. 4). Evaluation of other compounds thought to be upstream regulators of MMP expression (e.g., cyclooxygenase 2, prostaglandins, growth factors), other direct activators of MMPs (MT-MMP-1 28), and potential downstream effects on apoptosis or angiogenesis 23 remains to be completed. Also, correlation of MMP expression with recurrence and tumor death rates would be important with long-term follow-up. Treatment with MMP inhibitors for prevention or treatment of colorectal carcinomas remains experimental.
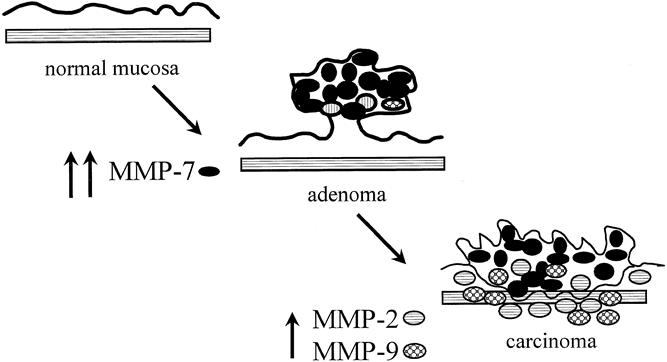
Figure 4. Matrix metalloproteinase (MMP) overexpression and progression of colorectal normal mucosa to adenoma and carcinoma.
Acknowledgments
The authors thank Jill Weingarten and Amanda Thompson for their excellent research nursing support and secretarial help, respectively.
Discussion
Dr. B. Mark Evers (Galveston, Texas): I rise to congratulate the authors on this well-done study assessing expression patterns of the matrix metalloproteinases 2, 7, and 9. The authors have assessed colonic adenomas, colon cancers, and adjacent normal mucosa and noted by real-time RT-PCR analysis that MMP-7 gene expression was increased in adenomas but did not increase further with cancer development. MMP-2 and MMP-9 were only elevated in the cancers. The data suggests a potential early role for MMP-7 in adenomas and then a later role for MMP-2 and MMP-9 in cancers.
This study provides information that further supports a potential role for the matrix metalloproteinases at various stages of carcinogenesis and will be useful for the consideration of possible inhibitors in the treatment of colon cancers. I have three questions for Dr. Heslin.
First, could you provide some information from either your own studies or the studies of others as to the localization of the expression of these genes in the normal colonic mucosa, adenomas, and cancers? For example, do you find expression in the colonocytes of the normal colon? Or, rather, is the expression pattern noted in the stroma-supported tissue?
In the same vein, in the colon cancers, is the expression in the colon cancer cells themselves or is it in the surrounding stromal tissues, such as fibroblasts contained within the tumor?
My second question has to do with whether the authors have assessed actual protein levels that nicely demonstrate increases in gene expression based upon RT-PCR data; however, mRNA and protein expression patterns do not always correlate. And it would be quite instructive to know whether protein levels of the MMPs are increased in a similar fashion as the mRNA.
Finally, it would be extremely beneficial to also look at MMP expression in metastatic lesions. Therefore, have you had the opportunity to examine expression patterns in patients where you could evaluate not only normal mucosa in the primary cancer but also metastasis to determine whether alterations in MMP expression levels are noted in the metastatic lesions compared with the primary cancers? This would lend further support to the suggestion that MMP-2 and MMP-9 may be more important in subsequent colon cancer metastasis.
I enjoyed the paper very much and thank the authors for the opportunity to comment on this nice study.
Dr. Andrew L. Warshaw (Boston, Massachusetts): I will not repeat the encomiums of this paper. It really is very nice work, and I will confine my comments to questions that this very intriguing study raises.
The first is, you measured RNA rather than DNA, and I wonder if you have any information on relative transcription that may impact on the levels that you are measuring.
Secondly, you have lumped adenomas together. I am not sure whether you are including tubular adenomas versus villous adenomas in this, and clearly there are different malignant potentials for different kinds of colon adenomas.
Third, you have used tissue fragments which were obtained immediately after colon resection, but these are relatively conglomerate pieces of tissue rather than microdissected for different segments, as best I can tell. And, therefore, the different elements perhaps are being put together. And, in particular, I am wondering how you are able to say that there was no relation to the degree of dysplasia, since you do not know the degree of dysplasia in the fragments that you are using.
Fourth, if you are unable to tell the degree of dysplasia or the difference between dysplasia and adenoma, I wonder if you can give us more information about the point of change that you indicate for the relative MMP distributions.
Next, you mentioned that MMP-2 and MMP-9 were increased at carcinoma stage in comparison with the adenoma stage. The difference in actual values, relative values, is 1.8 to 2.2 and 1.4 to 1.8. These small differences may not be truly different and may be accounted for by sample size alone. Certainly, the meaningfulness of that differential is unclear to me.
You also mentioned in your manuscript the tests of association that indicate that the MMP-2 and MMP-9 are coregulated by the MMP-7. I’m not sure that I understand why coexpression indicates or means coregulation.
Finally, if the effect of these different MMPs, that is, 2 and 9 versus 7, is seen to be different but all in some way have been, by other studies, related to the ability of a cancer to invade and metastasize, why is there no difference in stage of these tumors as you have seen them, the AJCC stage, relative to the different levels of MMPs that you have seen?
As with all great studies, many questions are raised, and I appreciate the chance to discuss this.
Dr. Alfred M. Cohen (Lexington, Kentucky): I appreciate the opportunity as a guest to comment. I would also congratulate the authors.
This may seem simple and straightforward, but as you can tell from the comments and the questions, it is a very complicated area. There are multiple collagens. These inhibitors are directed towards the type 4 collagen. It was mentioned at least 15 of these inhibitors. There are the so-called temps, which are inhibitors of the matrix metalloproteinases. We heard about where they are actually located, some in the normal stroma, even work has demonstrated some from monocytes. An incredibly complicated area.
There are multiple upstream determinants of the action; the whole COX-2 pathway probably is involved. I get the feeling that Dr. Urist thought this was an exciting area to work in and said, “Now let’s see, who in my group could possibly sort this out?” and picked the brightest, youngest person, who is Dr. Heslin, who is going to, I can see, spend the next decade trying to educate us. So I have just a couple of questions for you, Marty.
One is to repeat the question about the polyps. I know you only had 11 polyps, but I would like to know villous versus tubular adenoma. I know the dysplasia wasn’t relevant.
There are two strategies as you move ahead with the prognostic significance. I think most people have looked towards looking at logistic regression of Kaplan-Meier curves based on the stage. That is very difficult to sort out. I’d like to see you just come back with some follow-up information just as a group of perhaps stage 2 patients who are a group that recurred and those that didn’t recur to try to see if there is difference in that group.
And then there are some generic inhibitors of the matrix metalloproteinases, and the beauty of prevention studies and colorectal, you can look at polyp prevention. Are you aware of any studies that have looked at some of these famous or infamous inhibitors in terms of preventing polyps?
I appreciate the opportunity to speak to the group.
Dr. Martin J. Heslin (Birmingham, Alabama): Thank you for this opportunity to discuss the paper. I will talk about Dr. Evers’ comments first.
The localization of these MMPs, as Dr. Cohen alluded to, is very complex as to where they are actually located and which cells they come from. We did not look at specifically, in our study, either immunohistochemistry or in situ hybridization that looks at protein or at the specific RNA fragment in our studies, but other people have done that and do it a lot better than I could do it. So what those studies have demonstrated is that MMP-7 or matrilysin is located primarily on the tumor cell surface, which, as we alluded to in the presentation, gives the idea that it is an intrinsic process to the cancer cell, so specifically modulated by an altered pathway in the cancer cell. MMP-2 and -9, in at least three studies, have looked at localization, and in a very elegant study from Carrol Swallow which we alluded to in the manuscript, looked at activation of monocytes in a two-chamber setup, where you have cancer cells in a highly metastatic line and in the opposite chamber looked at MMP-2 and -9 expression by monocytes and demonstrated that there was a high rate of induction by monocytes of MMP-2 and -9 from metastatic cancer cells in a separate chamber, suggesting that monocytes do induce or can be induced to secrete these type of MMPs. It’s a process of tumor cells and surrounding stroma. Using that preliminary work, come up with a hypothesis to look at gene expression.
Dr. Evers, we also did not look at protein levels or activity. Really, that’s the next level of laboratory investigations, correlating expression, protein level, and activity, involving a whole other layer of laboratory expertise which I have not set up yet. And suffice it to say that hopefully we will head in that direction to give you more correlation in the future.
Now we have looked at MMP expression in metastatic lesions. One of the benefits of being clinically based and experimentally oriented is that when you operate on a patient with a primary colon cancer, you can also harvest metastatic tumor at the same time—normal liver, normal mucosa—and really set up models of progression of these MMPs or any other marker that you are interested in.
And I will answer your and Dr. Warshaw’s seventh question on relationship to stage. And that data is not shown here but talked about in the manuscript. There was no relationship between MMP expression of these three and the stage of the patient. Theoretically, you’d think, or it has been proposed, that MMPs progress from adenoma to noninvasive to node-positive to metastatic disease. And that is nicely packaged; however, we didn’t find that that was the case. There was no relationship between any of the MMPs suggesting that once a carcinoma is formed, whether it is stage 2, node-negative or whether it is node-positive or metastatic, once it is formed, these alloproteases are activated. Obviously, it is only 40 patients. We have about 150 more to analyze, and so we will get back to you on that.
Dr. Warshaw asked about relative DNA or what are the relative amounts of transcription. We didn’t look at the DNA and focused on what we thought was the pathway being turned on, and so focused on mRNA as opposed to the actual alterations in the DNA itself.
Both you and Dr. Cohen asked about villous versus tubular. Small numbers preclude us from separating that out further at this time.
The amount of dysplasia was looked and, again, small numbers showed no correlation. There is one prior study from Japan that has shown a relationship between MMP-7 to both size and dysplasia. Their study had about 35 patients in it, and so that may be a numbers issue, something we will look at in the future as we accrue more adenomas.
Dr. Warshaw asked about where the actual tumor cell separated from stroma, and you are really asking the question of laser capture microdissection. And looking at individual groups of cells and extracting the RNA and then using the real-time PCR with the Taqman to analyze that, and it is possible: we have not done that. It is one of the projects that we are teaming up with the laser capture microdissection people to try to do.
Coregulation versus coexpression. You are correct: we have no data suggesting coregulation, and coexpression is a more correct term. What we have shown is that—can I have the one backup slide that talks about association? Just click it one forward.
We did look at tests of association between these MMPs and then simply determining a linear relationship between when one goes up does the other go up, and is there a significant correlation between those two. What it did demonstrate was that there was an association between when MMP-7 increased, there was a trend towards an increase in MMP-9, but it showed that MMP-7 was coexpressed with MMP-2 highly significantly. So that with an increase in MMP-7, you did see an increase in MMP-2.
The next relationship that we examined was MMP-2 and MMP-9, and again a significant relationship when MMP-2 is increased in carcinomas, MMP-9 is also increased. You say, well, A increases B, B increases C, why doesn’t A increase C? It is just relatively small numbers, and statistically MMP-9 and MMP-7 didn’t reach statistical significance.
And, lastly, the question by Dr. Cohen, you asked about prognostic significance in stage 2 patients. Right question, harder population to answer it in because the mortality rates in that group of patients are relatively long, 25% at 5 years. Harder population to study. You need a lot of numbers to get an event-dense population. Stage 4 died faster and already had recurrence. I think that we will probably focus on stage 3 patients because we will have a large number of recurrences in a relatively short period of time.
The role of MMP inhibitors at the present time has not been very successful in many tumor types, and part of the problem is that most of the MMP inhibitors are broad-based in action they don’t inhibit specific MMPs. Inhibition of MMP-7, I think, would be a good place to start as an individual small molecule, because that would allow you to focus on what I think is the initiation process. The other problem with the generalized MMP inhibitors is that they produce significant side effects of arthralgias. And so in the prevention population, they are not really ready to be used for them.
I’d like to thank the Association for the opportunity to present. Thank you.
References
- 1.Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 1993; 104: 1535–1549. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER. Molecular genetics of colorectal cancer. Ann NY Acad Sci 1995; 768: 101–110. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767. [DOI] [PubMed] [Google Scholar]
- 4.Newell KJ, Witty JP, Rodgers WH, et al. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog 1994; 10: 199–206. [DOI] [PubMed] [Google Scholar]
- 5.Forster SJ, Talbot IC, Clayton DG, et al. Tumour basement membrane laminin in adenocarcinoma of rectum: an immunohistochemical study of biological and clinical significance. Int J Cancer 1986; 37: 813–817. [DOI] [PubMed] [Google Scholar]
- 6.Theret N, Musso O, Campion JP, et al. Overexpression of matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 in liver from patients with gastrointestinal adenocarcinoma and no detectable metastasis. Int J Cancer 1997; 74: 426–432. [DOI] [PubMed] [Google Scholar]
- 7.Poulsom R, Pignatelli M, Stetler-Stevenson WG, et al. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol 1992; 141: 389–396. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells [published erratum appears in Cell 1998 Jul 24;94(2): following 271]. Cell 1998; 93: 705–716. [DOI] [PubMed] [Google Scholar]
- 9.Zeng ZS, Huang Y, Cohen AM, et al. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol 1996; 14: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 10.Tsujii M, Kawano S, Dubois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997; 94: 3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342: 1946–1952. [DOI] [PubMed] [Google Scholar]
- 12.Yang VW, Geiman DE, Hubbard WC, et al. Tissue prostanoids as biomarkers for chemoprevention of colorectal neoplasia: correlation between prostanoid synthesis and clinical response in familial adenomatous polyposis. Prostaglandins Other Lipid Mediators 2000; 60: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MR, Wang K, Smith JB, et al. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochemy 2000; 278: 175–184. [DOI] [PubMed] [Google Scholar]
- 14.Witty JP, McDonnell S, Newell KJ, et al. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res 1994; 54: 4805–4812. [PubMed] [Google Scholar]
- 15.Shattuck-Brandt RL, Lamps LW, Heppner Goss KJ, et al. Differential expression of matrilysin and cyclooxygenase-2 in intestinal and colorectal neoplasms. Mol Carcinog 1999; 24: 177–187. [PubMed] [Google Scholar]
- 16.Takeuchi N, Ichikawa Y, Ishikawa T, et al. Matrilysin gene expression in sporadic and familial colorectal adenomas. Mol Carcinog 1997; 19: 225–229. [PubMed] [Google Scholar]
- 17.Bramhall SR. Stromal degradation by the malignant epithelium in pancreatic cancer and the therapeutic potential of proteolytic inhibition. J Hepatobiliary Pancreat Surg 1900; 5: 392–401. [DOI] [PubMed] [Google Scholar]
- 18.Heppner KJ, Matrisian LM, Jensen RA, et al. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol 1996; 149: 273–282. [PMC free article] [PubMed] [Google Scholar]
- 19.Pyke C, Ralfkiaer E, Tryggvason K, Dano K. Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol 1993; 142: 359–365. [PMC free article] [PubMed] [Google Scholar]
- 20.Swallow CJ, Murray MP, Guillem JG. Metastatic colorectal cancer cells induce matrix metalloproteinase release by human monocytes. Clin Exp Metastasis 1996; 14: 3–11. [DOI] [PubMed] [Google Scholar]
- 21.Dumas V, Kanitakis J, Charvat S, et al. Expression of basement membrane antigens and matrix metalloproteinases 2 and 9 in cutaneous basal and squamous cell carcinomas. Anticancer Res 1900; 19: 2929–2938. [PubMed] [Google Scholar]
- 22.Itoh T, Tanioka M, Yoshida H, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 1900; 58: 1048–1051. [PubMed] [Google Scholar]
- 23.Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase-2. Cancer Res 2000; 60: 5410–5413. [PubMed] [Google Scholar]
- 24.Sier CF, Kubben FJ, Ganesh S, et al. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer 1996; 74: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand K, Baker AH, Perez-Canto A, et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res 2000; 60: 5723–5730. [PubMed] [Google Scholar]
- 26.Hasegawa S, Koshikawa N, Momiyama N, et al. Matrilysin-specific antisense oligonucleotide inhibits liver metastasis of human colon cancer cells in a nude mouse model. Int J Cancer 1900; 76: 812–816. [DOI] [PubMed] [Google Scholar]
- 27.Haq M, Shafii A, Zervos EE, et al. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer Res 2000; 60: 3207–3211. [PubMed] [Google Scholar]
- 28.Mori M, Mimori K, Shiraishi T, et al. Analysis of MT1-MMP and MMP2 expression in human gastric cancers. Int J Cancer 1997; 74: 316–321. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Dr. Martin J. Heslin, Surgical Oncology, 1922 Seventh Ave. South, KB 321, Birmingham, AL 35243.
E-mail:marty.heslin@ccc.uab.edu
Accepted for publication December 2000.


