Abstract
Objective
To evaluate the University of Kentucky experience in treating acute intestinal ischemia to elucidate factors that contribute to survival.
Summary Background Data
Acute intestinal ischemia is reported to have a poor prognosis, with survival rates ranging from 0% to 40%. This is based on several reports, most of which were published more than a decade ago. Remarkably, there is a paucity of recent studies that report on current outcome for acute mesenteric ischemia.
Methods
A comparative retrospective analysis was performed on patients who were diagnosed with acute intestinal ischemia between May 1993 and July 2000. Patients were divided into two cohorts: nonthrombotic and thrombotic causes. The latter cohort was subdivided into three etiologic subsets: arterial embolism, arterial thrombosis, and venous thrombosis. Patient demographics, clinical characteristics, risk factors, surgical procedures, and survival were analyzed. Survival was compared with a collated historical series.
Results
Acute intestinal ischemia was diagnosed in 170 patients. The etiologies were nonthrombotic (102/170, 60%), thrombotic (58/170, 34%), or indeterminate (10/170, 6%). In the thrombotic cohort, arterial embolism accounted for 38% (22/58) of the cases, arterial thrombosis for 36% (21/58), and venous thrombosis for 26% (15/58). Patients with venous thrombosis were younger. Venous thrombosis was observed more often in men; arterial thrombosis was more frequent in women. The survival rate was 87% in the venous thrombosis group versus 41% and 38% for arterial embolism and thrombosis, respectively. Compared with the collated historical series, the survival rate was 52% versus 25%.
Conclusions
These results indicate that the prognosis for patients with acute intestinal ischemia is substantially better than previously reported.
Numerous surgical reports have indicated that acute intestinal ischemia (AII) is associated with a poor prognosis. 1–7 The basis of treatment for this condition traditionally emphasizes early diagnosis, resection of infarcted bowel, targeted surgical or nonsurgical restoration of blood flow to ischemic intestine, second-look laparotomy, and supportive intensive care. 8–12 Whether current diagnostic tests and these therapeutic strategies have had a favorable impact on survival is unknown. This is due in part to the paucity of data regarding outcome analyses during the past decade.
One aspect that influences survival is the cause of bowel ischemia, which can be classified as nonthrombotic or thrombotic events. Conditions that cause nonthrombotic mesenteric ischemia include low-flow states (e.g., cardiogenic shock, pancreatitis, sepsis, hypovolemia), mechanical causes (e.g., strangulated hernia, adhesive bands, intussusception), trauma, aortic dissection, drug-related causes (e.g., ergot overdose), and colon ischemia after abdominal aortic aneurysm repair. Specific thrombotic conditions include arterial embolization, arterial thrombosis, and mesenteric venous thrombosis. The purpose of this study was to determine whether early diagnosis and aggressive therapy have resulted in improved survival in patients with acute thrombotic intestinal ischemia compared with results reported during the past 30 years.
METHODS
The charts of all patients at the University of Kentucky Medical Center with the diagnosis of AII between May 1993 and July 2000 were reviewed and systematically analyzed with respect to pathogenesis, therapy, and outcome. Based on the clinical and surgical findings, patients were identified as having a nonthrombotic or a vascular thrombotic event (i.e., mesenteric arterial embolism, mesenteric arterial thrombosis, or mesenteric venous thrombosis). Regardless of cause, the study was limited to patients who had clinical evidence of AII confirmed by endoscopy, laparoscopy, or celiotomy. Demographic information, risk factors, time interval between onset of symptoms and diagnosis, vital signs at presentation, laboratory studies, diagnostic studies, and hospital length of stay (LOS) were recorded.
Patient age, vital signs, LOS, laboratory results, and death were analyzed using two-way analysis of variance to determine whether differences existed among diagnostic groups. When analysis of variance indicated a significant difference among these groups, a post hoc analysis using the Fisher plausible least significant difference post hoc test was performed to identify significant differences between specific groups. Differences in patient gender and coexistent clinical conditions were evaluated between groups using chi-square analysis. P < .05 was considered significant. The StatView V5.0 (SAS Institute, Inc., Cary, NC) software program was used for the statistical analyses.
This study was approved by the Institutional Review Board at the University of Kentucky.
RESULTS
During the study period, 170 patients with AII were identified. The causes were nonthrombotic (102/170, 60%), thrombotic (58/170, 34%), or indeterminate (10/170, 6%). In the nonthrombotic group, the mean age was 63.3 years (range 18–86). The male/female ratio was 1.9. The nonthrombotic causes were associated either with a low-flow state (71/102, 70%) or resection of an abdominal aortic aneurysm (31/102, 30%). In these categories, the ages were 60.9 and 68.8 years and the male/female ratio was 1.4 and 4.2, respectively.
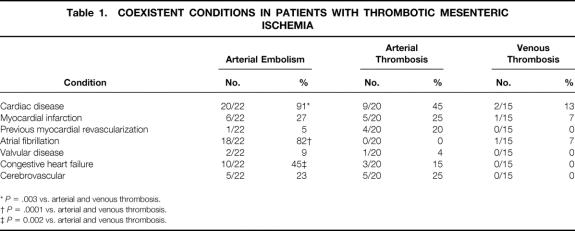
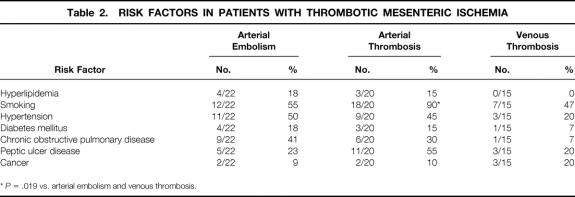
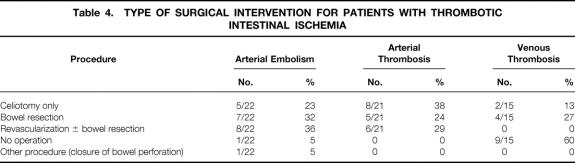
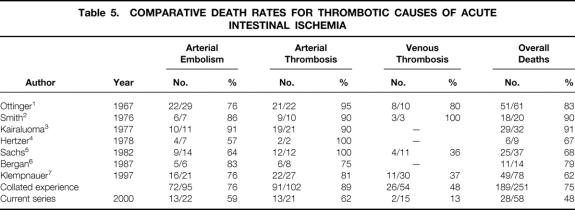
Patients with thrombotic causes of AII, the primary focus of this report, had a mean age of 60.9 years (range 20–91). These patients had a male/female ratio of 0.8. They were classified according to cause: arterial embolism (22/58, 38%), arterial thrombosis (21/58, 36%), and mesenteric venous thrombosis (15/58, 26%). In patients with arterial embolism, the average age was 75.5 ± 2.6 years (range 44–91), with two patients younger than 50 years. The male/female ratio was 0.8. The most common symptom was abrupt onset of abdominal pain (21/22, 95%), associated with nausea (10/22, 45%), vomiting (10/22, 45%), and diarrhea (4/22, 18%). The average time between onset of symptoms and presentation for definitive intervention was 2.4 ± 0.6 days. Half of these patients appeared for treatment within 24 hours of the onset of symptoms. Associated medical conditions are shown in Table 1. All but two of the patients had a history of significant heart disease. The source of the emboli was usually the heart and was most frequently related to atrial fibrillation. Other sources included a left ventricular mural thrombus (1/22, 5%) and an intraaortic balloon pump (1/22, 5%). With respect to risk factors, smoking and hypertension were observed in more than half the patients, more than a third of the patients had chronic obstructive pulmonary disease, and almost a quarter of the patients had a history of peptic ulcer disease and hyperlipidemia (Table 2). Laboratory studies revealed a marked leukocytosis, elevated blood urea nitrogen, mildly elevated serum creatinine, and an elevated serum lactate level (Table 3). Diagnostic studies performed in this patient group included angiography in 11 patients and a computed tomography scan in 5 patients. Other tests included diagnostic peritoneal lavage, transesophageal echocardiography, and celiotomy. With the exception of one patient who refused, all those with the diagnosis of arterial embolism underwent surgery (Table 4). Thrombolytic therapy was not attempted in any of these patients. Ten patients had a second-look laparotomy. The average LOS was 13.3 ± 2.6 days. If five patients were excluded for reasons of comfort care only, the LOS increased to 17.9 ± 2.7 days. The overall death rate was 59% (13/22), slightly less than that observed in the collated series as shown in Table 5.
Table 1. COEXISTENT CONDITIONS IN PATIENTS WITH THROMBOTIC MESENTERIC ISCHEMIA
*P = .003 vs. arterial and venous thrombosis.
†P = .0001 vs. arterial and venous thrombosis.
‡P = 0.002 vs. arterial and venous thrombosis.
Table 2. RISK FACTORS IN PATIENTS WITH THROMBOTIC MESENTERIC ISCHEMIA
*P = .019 vs. arterial embolism and venous thrombosis.
Table 3. VITAL SIGNS AND LABORATORY STUDY RESULTS AT THE TIME OF DIAGNOSIS
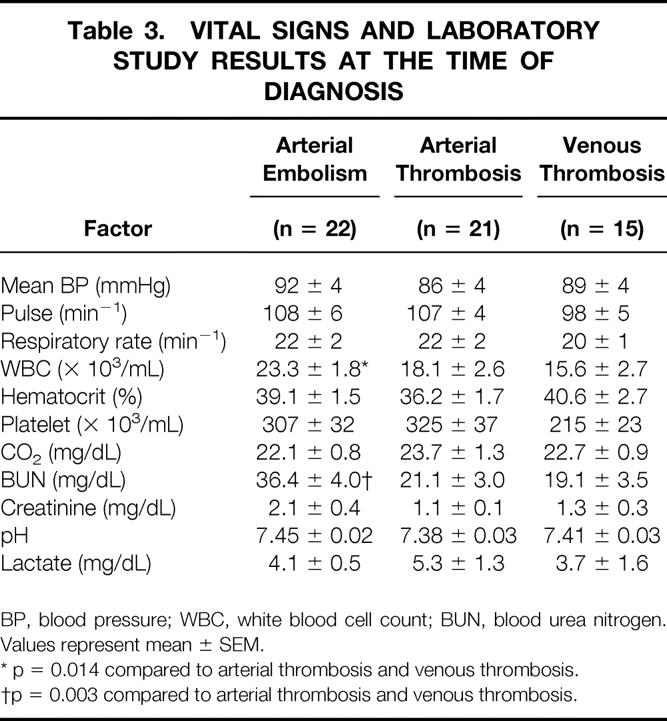
BP, blood pressure; WBC, white blood cell count; BUN, blood urea nitrogen. Values represent mean ± SEM.
* p = 0.014 compared to arterial thrombosis and venous thrombosis.
†p = 0.003 compared to arterial thrombosis and venous thrombosis.
Table 4. TYPE OF SURGICAL INTERVENTION FOR PATIENTS WITH THROMBOTIC INTESTINAL ISCHEMIA
Table 5. COMPARATIVE DEATH RATES FOR THROMBOTIC CAUSES OF ACUTE INTESTINAL ISCHEMIA
Patients with arterial thrombosis were on average 59.0 ± 2.7 years old (range 41–80); one third (7/21) were 50 years of age or younger. The male/female ratio was 0.3. Time from onset of acute symptoms to presentation was 4.4 ± 1.0 days, although six patients came to treatment within 24 hours of the onset of symptoms. The most common symptom, sudden onset of abdominal pain, was observed in all patients and was associated with nausea (13/21, 62%), vomiting (13/21, 62%), diarrhea (7/21, 33%), postprandial pain (4/21, 19%), and weight loss (2/21, 10%). Associated medical conditions for these patients are shown in Table 1. Half of the patients had a cardiac history. With respect to risk factors, smoking was observed in almost all patients, hypertension and peptic ulcer disease in half of the patients, and chronic obstructive pulmonary disease in a third of the patients (see Table 3). Similar to patients in the arterial embolism group, patients with arterial thrombosis had a leukocytosis and an increase in serum lactate level, but a mild elevation in blood urea nitrogen (see Table 3). Diagnostic studies performed in this patient group included angiography in eight patients and computed tomography scanning in seven. All patients underwent surgery (see Table 4), with intraoperative revascularization accomplished by either an aorta-to-superior mesenteric artery bypass graft or thrombectomy. Two patients were treated with thrombolytic therapy before surgery. One patient subsequently required a bowel resection; the other patient was found to have massive bowel necrosis at laparotomy, precluding further intervention. All but one of the patients who underwent revascularization also underwent bowel resection. Four of seven patients who underwent revascularization died; two of seven died who had bowel resection only. Five patients were returned to the operating room for a second-look laparotomy. The average LOS was 15.0 ± 4.4 days. If eight patients were excluded for comfort care only, the LOS increased to 22.9 ± 5.5 days. The death rate for the arterial thrombosis group was 62%, which was 30% lower than the reported collated mortality rate (see Table 5).
In the cohort of patients with mesenteric venous thrombosis, the average age was 43.0 ± 3.2 years (range 20–63). Almost three quarters of the patients (11/15) were younger than 50 years and almost half (7/15) were younger than 40 years. The male/female ratio was 2.8. The most common symptom was abdominal pain (14/15, 93%), associated with nausea (10/15, 67%), vomiting (9/15, 60%), diarrhea (2/15, 13%), hematemesis (2/15, 13%), and hematochezia (2/15, 13%). On average, patients had symptoms for 15.0 ± 6.0 days before admission. Three patients were admitted within 24 hours of the onset of symptoms. Associated medical conditions are shown in Table 1. Two patients had a history of cardiac disease and one had atrial fibrillation. With respect to risk factors, smoking was observed in half of the patients, and peptic ulcer disease and cancer were observed in one fifth of patients (see Table 2). A hypercoagulable profile was obtained only in patients with a diagnosis of mesenteric venous thrombosis. Of those tested, a third were found to have a protein C or protein S deficiency. Patients with mesenteric venous thrombosis had a moderate leukocytosis and an elevated serum lactate level (see Table 3). The diagnosis of mesenteric venous thrombosis in most patients (12/15) was made on the basis of an abdominal computed tomography scan (Fig. 1). Other diagnostic studies included endoscopy (n = 3), angiography (n = 2), and duplex ultrasound (n = 3). Most patients were treated with anticoagulation without surgery (see Table 4). In the two patients who underwent celiotomy without bowel resection, the bowel was ischemic but not infarcted. In no patient was an attempt made to perform a mesenteric venous thrombectomy. Two patients had thrombolytic therapy (urokinase) selectively infused into the superior mesenteric artery; both survived. Four patients were returned to the operating room for a second-look laparotomy. The average LOS was 14.9 ± 3.3 days. In two patients who died, one had an anastomotic leak after pancreatoduodenectomy. In the other, diagnosis was made 45 days after onset of symptoms when generalized peritonitis from bowel perforation had developed. The death rate for the venous thrombosis group was 13%, almost five times less than that observed in the collated series (see Table 5).
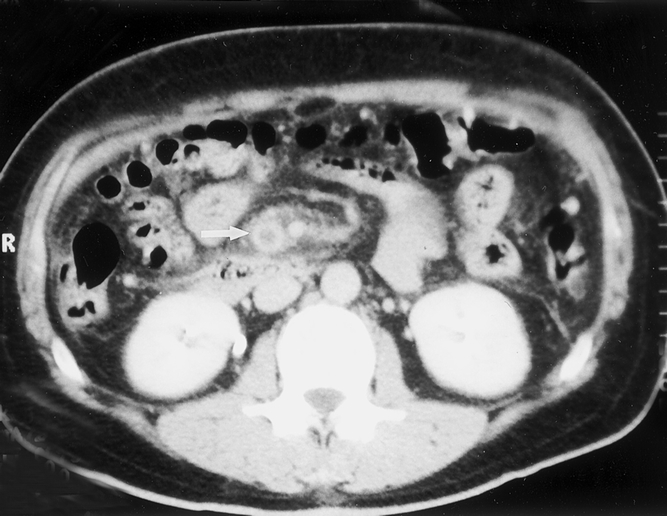
Figure 1. Abdominal computed tomography scan in a patient with mesenteric venous thrombosis. The arrow points to the superior mesenteric vein. The vein is distended with intraluminal thrombus. The venous wall is enhanced with contrast as a result of arterial filling of the venous vasa vasorum and gives a halo effect.
DISCUSSION
This report makes several important observations. Approximately a third of AII cases were due to a vascular thrombotic etiology, an incidence less than previously reported. However, one quarter of these patients had mesenteric venous thrombosis, a rate higher than expected. 13,14 Arterial thromboembolic events affected patients over a wide age range who were characterized by a high incidence of smoking, hypertension, and a history of cardiac disease. However, those with venous thrombosis were predominantly men younger than 50 years. Most importantly, the prognosis for patients with AII in the University of Kentucky analysis was substantially better than previously reported, a finding that supports the hypothesis that early diagnosis and aggressive treatment 14,15 can have a salutary effect on survival.
Historically, AII portends a poor prognosis, with death rates ranging from 60% to 100%. 1–7 The surgical treatment of the condition is well established and consists of revascularization and/or resection of nonviable bowel. 8–12 However, successful treatment depends on the ability to make the correct diagnosis of the underlying condition. Giulini et al 16 have shown a correlation between prompt diagnosis of AII and survival. However, because of the nonspecific nature of symptoms during the early phase of the disease process, diagnosis is often delayed. 17 In these patients, the diagnosis is entertained only after extensive infarction has developed, and aggressive surgical intervention may be futile. This led Taylor et al 18 to state that “the mortality rate for patients with acute intestinal ischemia will probably always remain high.” Although this bleak expectation is justified based on historical data, our current analysis does not substantiate this conclusion.
One of the most important factors relative to successful outcome is early diagnosis. Intestinal ischemia is found in 1 or 2 of 1,000 hospital admissions, 14 or 1% to 2% of all patients admitted with gastrointestinal diseases. 19 Because of its relative rarity, physicians may not recognize the early nonspecific signs and symptoms and thereby attribute a patient’s complaints to other causes. 20 For example, a high percentage of patients in the current study gave a history of peptic ulcer disease, seen especially in patients with arterial thrombosis. This finding may, in part, have contributed to the delay in diagnosis seen in this cohort of patients. Such a delay can often lead to therapy that ultimately proves to be without benefit. 21 Further, AII is thought to affect primarily the elderly. 17 These patients may have atypical presentations or may be confused and unable to articulate their complaints, further confusing the clinical picture. 22 As the current report shows, however, younger patients are also affected, and failure to recognize that patients over a wide age range are at risk for intestinal ischemia will delay diagnosis. Some authors have advocated early arteriography in patients with suspected AII both for diagnostic purposes and to help optimize surgical treatment for the underlying arterial pathology 23 (Fig. 2).
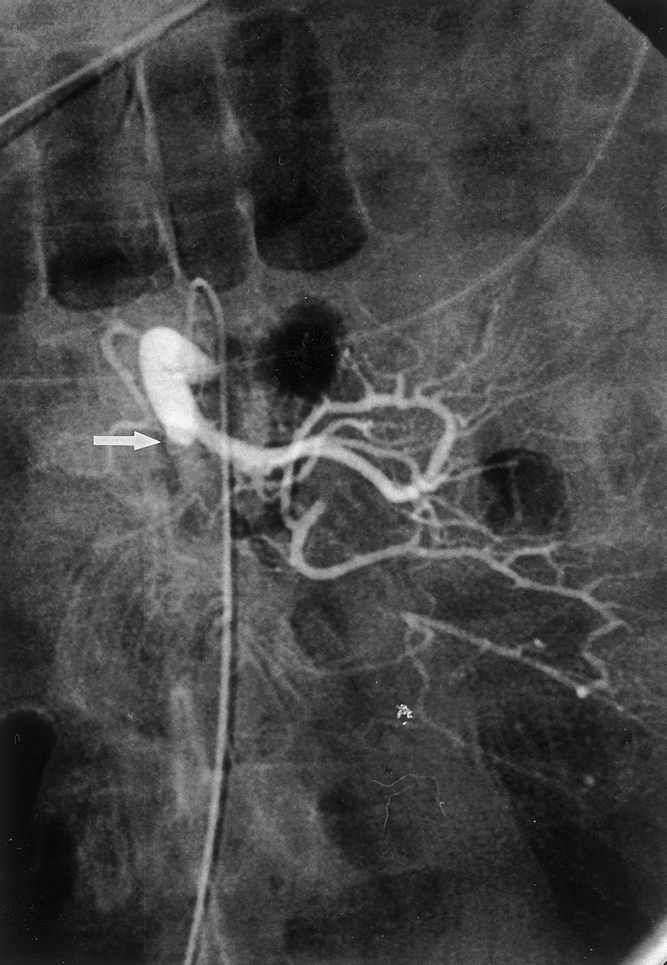
Figure 2. Selective arteriogram of the superior mesenteric artery that shows findings consistent with an embolus (arrow). The proximal branches are perfused; the distal artery is occluded.
Aggressive management is also crucial for successful outcome. 8,10,14,19 In general, treatment involves fluid resuscitation, invasive hemodynamic monitoring, prophylactic antibiotics, and systemic anticoagulation with heparin. These principles are especially important in patients with mesenteric venous thrombosis. Heparin is necessary to prevent propagation of thrombus, and long-term anticoagulation with warfarin is needed to prevent recurrence, which is reported in more than one third of patients. 24 Close monitoring of fluid status is required because in patients with venous thrombosis, significant bowel congestion can develop; this in turn can lead to sequestration of large volumes of fluid. 25 If undertreated, this process can progress to hemoconcentration, hypovolemia, and shock, exacerbating the ischemia. Surgery is reserved for patients in whom signs of bowel infarction develop; if required, these patients may need wide resection. 26 There are anecdotal reports of venous thrombectomy, but this has not shown improved outcome and is generally not recommended. 10,25 In contrast, patients with arterial causes of AII should undergo emergent surgery to revascularize ischemic bowel and resect infarcted bowel. Revascularization can be accomplished by extraction of thrombus or embolus and/or bypass of the occlusive lesion. After revascularization, a conservative approach to bowel resection is warranted to preserve as much intestine as possible. In all patients, bowel that is not infarcted but of questionable viability should not be resected; in these cases, a second-look laparotomy is indicated. 27 In the current report, bowel viability was assessed by visual inspection (color, peristaltic activity), presence of Doppler signals on the antimesenteric border, and fluorescein uptake as viewed under ultraviolet illumination. Nonviable bowel can be safely resected at the second laparotomy.
With the current interest in the endovascular treatment of vascular disease, the role of endovascular techniques, such as thrombolysis and percutaneous transluminal angioplasty, to restore bowel perfusion in patients with AII needs to be considered. In the current report, these methods of treatment were only occasionally used. However, recent reports have described the utility of these less invasive techniques for the treatment of intestinal ischemia. 28,29 The ability to institute immediate treatment in the form of thrombolysis at the time of diagnostic angiography holds some attraction. However, although this may more rapidly restore blood flow to the bowel in some patients, the time needed for lysis of thrombus is variable, and bowel viability cannot be accurately assessed without laparotomy. Reliance on thrombolysis to restore bowel perfusion may in fact result in significant delays, exacerbating bowel ischemia. Consequently, what was ischemic but potentially viable bowel may progress to bowel infarction while awaiting thrombolysis. Currently, surgical revascularization with concurrent assessment of bowel viability should be considered the standard approach to patients with AII from arterial thromboembolism. Significant clinical judgment will be required to select appropriate patients for endovascular treatment of AII.
Because the specific cause of intestinal ischemia has a direct effect on treatment and outcome, it would be helpful to the clinician if patient characteristics associated with specific causes could be identified. Although this study found few clinical characteristics to serve as guidelines, it was noted that patients with venous thrombosis tended to be younger; those with arterial embolism were the oldest. Patients with arterial embolism invariably had a significant heart history. Abdominal pain was nearly a uniform finding, but in those with arterial causes of intestinal ischemia, the pain was more pronounced, leading to earlier diagnosis. Unfortunately, regardless of cause, no laboratory or clinical features could be identified that specifically differentiated patients among diagnostic groups or predicted survival.
In view of the improved outcome, there are recognized limitations to the current review. The number of patients in each diagnostic category was small, although larger than most of the individual series available for analysis. 1–7 Although the favorable outcomes noted in the University of Kentucky series may be related to a population skewed toward patients with milder forms of intestinal ischemia, the coexistent conditions and risk factors suggest that the patient population is representative. Also, a retrospective comparative analysis does not take into consideration that treatment rendered for specific patients was carried out by multiple physicians and that each physician tailored the treatment to the specific findings and needs of the patient. Finally, this study does not include a cost analysis. It was noted that patients who survived had an average LOS of 3 weeks, half of which was spent in the intensive care unit. Given the severity of this illness, future studies are needed to determine whether additional financial and human resources necessary for improved survival are justified.
This study does, however, represent a contemporary analysis of patients with AII secondary to thrombotic causes who were treated according to accepted guidelines and in whom survival was substantially better than previously reported. This improvement was observed with respect to overall survival as well as in each of the thrombotic categories. Despite the lower death rate, however, there is still opportunity for improvement. The two deaths observed in patients with mesenteric venous thrombosis were either preventable or not specifically related to the mesenteric ischemic event. In the group of patients with arterial causes of AII, 13 patients, 5 in the arterial embolism group and 8 in the arterial thrombosis groups, were found to have extensive bowel infarction at the time of laparotomy and received only comfort care treatment. By definition, the diagnosis in these patients was delayed. Had these patients been excluded and the death rate based on patients who were aggressively treated, the death rate would have been lower (i.e., 47% and 38% for patients with arterial embolism and arterial thrombosis, respectively). These results continue to show the importance of early diagnosis and aggressive treatment, and also emphasize that future survival rates should be substantially better than the prognosis previously reported.
Acknowledgments
The authors thank Michael Donnelly, PhD, for the statistical analysis of the data and Caren Mulford, MA, and Linda Macleery for their assistance in the preparation of this manuscript.
Discussion
DR. ALI F. ABURAHMA (Charleston, West Virginia): I wish to thank Dr. Endean for sending me this paper to review in advance. Acute intestinal ischemia is a frequently misdiagnosed disorder and carries a high mortality rate, as indicated by the presenter. This is a well-written paper describing 7 years’ experience of treating patients with acute intestinal ischemia, 60% of which were secondary to nonthrombotic states and 34% due to thromboembolic events. This paper correctly emphasized the early diagnosis and aggressive treatment to minimize perioperative morbidity and mortality. I have the following questions for Dr. Endean:
What are your workup priorities in these patients to achieve early diagnosis? Do you see any role in using color duplex ultrasound in these patients in spite of the presence of excessive gas? What methods did you use to check bowel viability in surgery? Can you describe the role of lysis/PTA/stenting in treating patients with acute intestinal ischemia? As a matter of fact, few selected patients in our center were treated with endovascular means (e.g., PTA/stenting with percutaneous AngioJet embolectomy) successfully. Have you seen bowel hemorrhages secondary to the use of lysis?
I want to thank the Southern Surgical for the honor of discussing this paper.
DR. R. NEAL GARRISON (Louisville, Kentucky): Dr. Baker, Secretary Townsend. I want to congratulate Dr. Mentzer and colleagues on this extensive report of a very complex clinical problem of acute intestinal ischemia. They report improved survival in all categories of acute ischemia compared to other reports in the literature. I can make one observation about this literature comparison, however. Two early reports by Ottinger in 1967 and Smith in 1976 list 80% and 100% mortality rate. And if you discard those, I believe that other more recent reports listed are comparable to your outcomes in the venous thrombosis group. I have several comments and questions that might help the practicing surgeon when he deals with this entity.
You attribute improved outcome to early diagnosis and aggressive management, but you do not report in your manuscript a time variable such as onset of symptoms to the diagnosis that would support that conclusion. Do you have such data that supports that early diagnosis leads to better improvement and outcome?
Are there certain intraoperative techniques that your group favors for assessment of bowel viability? In those patients where a second-look operation was done, was additional bowel resected or was your initial assessment accurate in predicting bowel survival?
At the initial operative intervention, were the bowel ends following resection exteriorized or simply closed with a planned second-look to establish viability and reestablish intestinal continuity?
Finally, I cannot help but ask if you used enteral or parenteral nutrition in the postoperative period. Your arterial embolus and thrombosis groups represent a human intestinal ischemia reperfusion injury similar to the mouse model that Dr. Kudsk reported on earlier today. Did those patients that were successfully revascularized demonstrate a clinical picture of lung failure? If so, this observation would help to validate animal models of intestinal ischemia/reperfusion as a precursor to organ system failure.
I thank the Association for the privilege of the floor.
DR. RICHARD BELL (Columbia, South Carolina): Thank you very much, Dr. Baker, Dr. Townsend, President Aust, members, and guests. When I first got this paper, I thought the point was going to be that things got better in Lexington after I left the University of Kentucky, particularly with reference to this problem. But after reading the manuscript carefully, it appears to me that the authors suggest that early diagnosis and aggressive resuscitation and timely operative intervention can result in survival rates that approach 70% or so. This is the largest series of which I am aware and one that reports some of the best outcomes. Early diagnosis may be the key to this. And my question is similar to those who have come before me. What have you learned over this almost 84-month period that helps me as a practicing surgeon make the diagnosis earlier? Is there anything in the physical examination, the history, the laboratory that gets me to consider angiography or operative intervention at an earlier date?
A second question is what do you see the role and the future role of endovascular manipulation and endovascular pharmacotherapy in these disease processes? Seidel in the Journal of Vascular Surgery in August of 1999 had an animal model that suggested measuring the basic electrical rhythm of the small bowel might be a key in the early recognition of these problems. Do you have any thoughts about that?
And the last question that I have for you pertains to the second-look celiotomy. Would you share your findings at the second-look with me? My personal experience suggests that this procedure has not been therapeutic in most of my patients. If the ischemic process has progressed, these patients are usually dead within 48 hours. And those who have corrected their base deficits have not required further resection. Your comments would be appreciated.
I congratulate the work from those from my alma mater and am grateful to the Association for the privilege of discussing. Thank you.
DR. HARVEY J. SUGERMAN (Richmond, Virginia): I rise to question the two groups. First, the low-flow nonthrombotic-state patients. Did any of these patients have an acute abdominal compartment syndrome that could have led to their ischemic event? In particular, for example, you had a high incidence of frequency of this in your abdominal aortic aneurysm patients. And as pointed out by Dr. Kron at our sister institution in Virginia, at the University of Virginia, many of these patients will go on to develop an acute abdominal compartment syndrome, which then, we have found, can lead to acute intestinal ischemia. And so perhaps, looking at the preventive phase of this, should perhaps some of these patients not have had their abdomen closed after the procedure?
And then with regards to the venous thrombosis patients, did you look at obesity as one of the possible factors leading to venous thrombosis in these patients, as a chronic increase in intraabdominal pressure could lead to venous thrombosis as it can lead to thrombosis in the veins and the legs in some patients?
Thank you.
DR. LEWIS M. FLINT, JR. (Tampa, Florida): Thank you, Dr. Baker. I’d like to congratulate Dr. Endean for a very nice manuscript, which he was kind enough to send me in advance, and congratulate him on his endurance for having put up with the fact that Dr. Mentzer had to get up here and present his work, which is a peculiar characteristic of the Southern Surgical that young people have to endure. I have subjected Dr. Mentzer to that particular form of suffering, and I know that he appreciates the opportunity to return the favor to other people.
I have two questions with regard to your work that have to do with the critical care aspects of the management of these patients, one that has been touched on by previous discussers, having to do with the second-look procedure. I agree with Rick Bell, that I looked back over about a dozen patients over the past 2 years that we have managed in conjunction with our vascular service, where we have done second-look procedures following the diagnosis of mesenteric ischemia. And only one of those patients required further intestinal resection. And, unfortunately, in that one patient, there were no symptoms of further intestinal ischemia, no clinical symptoms. And we have adopted a management approach to these patients, which is one that forces you to go back, in that we do not anastomose the intestine and we don’t close the abdominal wall, which makes certain that you will go back, regardless of the clinical presentation of the patient, and hopefully avoid that occasional patient who will have the further ischemia without clinical symptomatology.
I’d like to ask you a question about the patients with the low-flow states. These patients tend to be temporally distributed, in our experience. They usually emerge on a Friday afternoon from the medical intensive care unit. And I’d like to ask you, if you have a patient that you think is a candidate for this particular problem, do you have any effective preventive therapies that might forestall the development of frank intestinal ischemia?
Thank you.
DR. ERIC D. ENDEAN (Lexington, Kentucky): I’d like to thank the discussants for their insightful comments, and I will try and answer them in order. Dr. AbuRhama, you asked what workup we used to achieve the early diagnosis. We would recommend that liberal use of angiography be undertaken. We also found that we were frequently able to make the diagnosis on the basis of CT scanning. Our radiologists are somewhat sensitized to this diagnosis and can pick out fairly early signs on CT scans that lead us to the diagnosis of ischemic bowel or infarcted bowel. We have used duplex ultrasound in some cases but have not found it particularly useful, especially because of the reasons you brought up, the large amount of bowel gas that is present. It also requires the technician to be present, and in our hospital, they are not available at night and on weekends, and often these patients come in at that time.
We have used thrombolysis and stenting in some patients, particularly those that we feel have arterial thrombosis. We have had some success, but I really can’t comment on that because it is a very limited number, and for that reason as well we have not seen bowel hemorrhage. However, I believe that that is a potentially significant problem.
Dr. Garrison, you asked about the time variable from onset of symptoms until treatment. This varied widely with diagnoses. It was approximately 2 days for those that had ischemia from arterial embolism, about 4 days for those that had ischemia from arterial thrombosis, and about 2 weeks, on average, for those with venous thrombosis. That time period was from when the symptoms began until they presented in our hospital for definitive therapy.
Our technique for assessing bowel viability intraoperatively primarily relies on fluorescein but also the appearance of a bowel at the time of surgery and after it has been revascularized.
We do incorporate second-look laparotomy, as has been asked by a number of discussants. We have found on some occasions that it has been helpful, that we do resect additional bowel. Our approach is that we try and preserve as much bowel as possible, and so in cases where there is patchy necrosis or areas that we feel are not completely infarcted, we will leave those behind, staple off the bowel ends, and come back in 24 or 36 hours.
We have a strong predilection to enteral nutrition, so most of these patients are started fairly early on feeding. Most of these patients receive a masoenteric feeding tube at the time of their operation. We have seen a number of patients that have pulmonary failure after this operation, but I’m not sure that I can attribute that specifically to successful revascularization. Lung failure, pneumonia, ARDS, if you will, has been a relatively common problem in these patients.
Dr. Bell, what have we learned that helps us make the diagnosis earlier? That is a difficult question to answer. I think that from our experience, since we see a large number of patients with all, we have a heightened awareness that this is a potential diagnosis. It is something that our residents have at the top of their differential when a patient comes in with abdominal pain. We do, as I mentioned, rely on angiography and CT scanning fairly extensively.
Dr. Sugerman, in the low-flow cases, I am not aware that there are any of our patients that developed intestinal ischemia because of an acute abdominal compartment syndrome. A number of these patients, however, did not have their abdomen closed and were treated with an open abdomen to prevent that problem postoperatively. There is a high incidence of patients in this series who had ischemic bowel in association with repair of abdominal aortic aneurysm, and I think this reflects some of the previous work from our institution. We are very aware that this can happen, and use sigmoidoscopy fairly liberally. A number of patients are found to have intestinal ischemia, primarily mucosal ischemia and are included in that group.
We did not note that obesity was a factor in the venous thrombosis group.
Dr. Flint, I believe I did address some of the issues regarding a second-look procedure. We do incorporate that precisely for the reason that you indicated, that many of these patients may have ongoing ischemia or have an area of infarcted bowel without further clinical symptoms. We determine at the time of the first operation whether or not they should have the second-look.
For those patients with the low-flow state, I am not sure that I have a good answer for you for our medicine colleagues. We would recommend angiography, and if there is not a thrombotic event in a patient with what is presumed to be intestinal ischemia, would recommend infusion of vasodilators.
I’d like to thank the Association for the opportunity to present our work.
References
- 1.Ottinger LW, Austen WG. A study of 136 patients with mesenteric infarction. Surg Gynecol Obstet 1967; 124: 251–261. [PubMed] [Google Scholar]
- 2.Smith JS, Patterson LT. Acute mesenteric infarction. Am Surg 1976; 42: 562–567. [PubMed] [Google Scholar]
- 3.Kairaluoma MI, Kärkölä P, Heikkinen E, et al. Mesenteric infarction. Am J Surg 1977; 133: 188–193. [DOI] [PubMed] [Google Scholar]
- 4.Hertzer NR, Beven EG, Humphries AW. Acute intestinal ischemia. Am Surg 1978; 44: 744–749. [PubMed] [Google Scholar]
- 5.Sachs SM, Morton JH, Schwartz SI. Acute mesenteric ischemia. Surgery 1982; 92: 646–653. [PubMed] [Google Scholar]
- 6.Bergan JJ, McCarthy WJ, Flinn WR, et al. Nontraumatic mesenteric vascular emergencies. J Vasc Surg 1987; 5: 903–909. [DOI] [PubMed] [Google Scholar]
- 7.Klempnauer J, Grothues F, Bektas H, et al. Long-term results after surgery for acute mesenteric ischemia. Surgery 1997; 121: 239–243. [DOI] [PubMed] [Google Scholar]
- 8.Mansour MA. Management of acute mesenteric ischemia. Arch Surg 1999; 134: 328–330. [DOI] [PubMed] [Google Scholar]
- 9.Whitehill TA, Rutherford RB. Acute intestinal ischemia by arterial occlusions: optimal management to improve survival. Semin Vasc Surg 1990; 3: 149–156. [Google Scholar]
- 10.Kispert JF, Kazmers A. Acute intestinal ischemia caused by mesenteric venous thrombosis. Semin Vasc Surg 1990; 3: 157–171. [Google Scholar]
- 11.Montgomery RA, Venbrux AC, Bulkley GB. Mesenteric vascular insufficiency. Curr Probl Surg 1997; 34: 945–1025. [DOI] [PubMed] [Google Scholar]
- 12.McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am 1997; 77: 307–318. [DOI] [PubMed] [Google Scholar]
- 13.Lipski DA, Earnst CB. Visceral ischemic syndromes. In: Moore WS, ed. Vascular surgery: a comprehensive review. Philadelphia: Saunders; 1998: 543–554.
- 14.Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery 1993; 114: 489–490. [PubMed] [Google Scholar]
- 15.Kaleya RN, Sammartano RJ, Boley SJ. Aggressive approach to acute mesenteric ischemia. Surg Clin North Am 1992; 72: 157–182. [DOI] [PubMed] [Google Scholar]
- 16.Giulini S, Bonardelli S, Cangiotti L, et al. Factors affecting prognosis in acute intestinal ischemia. Int Angiol 1987; 6: 415–420. [PubMed] [Google Scholar]
- 17.Rush DS, Levy PJ, Haynes JL. Acute embolic and thrombotic mesenteric ischemia. In: Ernst CB, Stanley JC, eds. Current therapy in vascular surgery. St. Louis: Mosby; 1995: 693–697.
- 18.Taylor LM, Moneta GC, Porter JM. Treatment of acute intestinal ischemia caused by arterial occlusions. In: Rutherford RB, ed. Vascular surgery. Philadelphia: Saunders; 2000: 1512–1519.
- 19.Schneider TA, Longo WE, Ure T, et al. Mesenteric ischemia: acute arterial syndromes. Dis Colon Rectum 1994; 37: 1163–1174. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Shah RC, Lee ST. Acute mesenteric vascular occlusion: a review of thirty-two patients. Surgery 1975; 78: 613–617. [PubMed] [Google Scholar]
- 21.Slater H, Elliott DW. Primary mesenteric infarction. Am J Surg 1972; 123: 309–311. [DOI] [PubMed] [Google Scholar]
- 22.Finucane PM, Arunachacam T, O’Dowd J, et al. Acute mesenteric infarction in elderly patients. J Am Geriatr Soc 1989; 37: 355–358. [DOI] [PubMed] [Google Scholar]
- 23.Bergan JJ. Diagnosis of acute intestinal ischemia. Semin Vasc Surg 1990; 3: 143–148. [Google Scholar]
- 24.Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg 1994; 20: 688–697. [DOI] [PubMed] [Google Scholar]
- 25.Harward TRS, Seeger JM. Mesenteric venous thrombosis. In: Ernst CB, Stanley JC, eds. Current therapy in vascular surgery. St. Louis: Mosby; 1995: 710–713.
- 26.Kazmers A. Intestinal ischemia caused by venous thrombosis. In: Rutherford RB, ed. Vascular surgery. Philadelphia: Saunders; 2000: 1524–1531.
- 27.Levy PJ, Krausz MM, Manny J. Acute mesenteric ischemia: improved results: a retrospective analysis of ninety-two patients. Surgery 1990; 107: 372–380. [PubMed] [Google Scholar]
- 28.Loomer DC, Johnson SP, Diffin DC, et al. Superior mesenteric artery stent placement in a patient with acute mesenteric ischemia. J Vasc Intervent Radiol 1999; 10: 29–32. [DOI] [PubMed] [Google Scholar]
- 29.VanDeinse W, Zawacki JK, Phillips D. Treatment of acute mesenteric ischemia by percutaneous transluminal angioplasty. Gastroenterology 1986; 91: 475–478. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Eric D. Endean, MD, 800 Rose St., Department of Surgery, Lexington, KY 40536.
E-mail: edende0@pop.uky.edu
Accepted for publication December 2000.