Abstract
Objective
To determine whether the beneficial effects of growth hormone persist throughout the prolonged hypermetabolic and hypercatabolic response to severe burn.
Summary Background Data
The hypermetabolic response to severe burn is associated with increased energy expenditure, insulin resistance, immunodeficiency, and whole body catabolism that persists for months after injury. Growth hormone is a potent anabolic agent and salutary modulator of posttraumatic metabolic responses.
Methods
Seventy-two severely burned children were enrolled in a placebo-controlled double-blind trial investigating the effects of growth hormone (0.05 mg/kg per day) on muscle accretion and bone growth. Drug or placebo treatment began on discharge from the intensive care unit and continued for 1 year after burn. Total body weight, height, dual-energy x-ray absorptiometry, indirect calorimetry, and hormone values were measured at discharge, then at 6 months, 9 months, and 12 months after burn. Results were compared between groups.
Results
Growth hormone subjects gained more weight than placebo subjects at the 9-month study point; this disparity in weight gain continued to expand throughout the remainder of the study. Height also increased in the growth hormone group compared with controls at 12 months. Change in lean body mass was greater in those treated with growth hormone at 6, 9, and 12 months. Bone mineral content was increased at 9 and 12 months; this was associated with higher parathormone levels.
Conclusions
Low-dose recombinant human growth hormone successfully abates muscle catabolism and osteopenia induced by severe burn.
The hypermetabolic response to severe trauma is associated with increased systemic energy expenditure, peripheral insulin resistance, immunodeficiency, and marked whole body catabolism. These systemic derangements are most profound after severe burn. Classically, hypermetabolism associated with injury was thought to recede with closure of wounds and healing of bone and soft tissue injury. 1,2 In our long-term follow-up of severely burned children, however, we found that hypermetabolism did not abate after full healing of burn wounds; in fact, we found that children burned over 40% total body surface area (TBSA) undergo muscle protein catabolism for at least 9 months 3 and growth arrest for at least 2 years after injury. 4 The clinical result of this persistent pathology is wasting of musculature at a time when strength reserves would be of benefit to assist with recovery, rehabilitation, and reintegration into society.
Growth hormone is known to be a potent anabolic agent and salutary modulator of posttraumatic metabolic responses. 5 After severe burn, it has been shown to decrease whole body catabolism, 6 improve muscle protein synthesis, 7 accelerate wound healing, 8,9 attenuate prolonged hyperactivity of the hepatic acute-phase response, 10,11 and promote linear growth. Its side effects when used in burned children are well characterized, and it has been shown to be a safe pharmacotherapeutic adjunct to standard excisional therapy after severe burn. 12,13
The purpose of this study was to determine whether the effects of growth hormone after severe burn persist throughout the prolonged hypermetabolic and hypercatabolic response to severe burn. Specifically, our hypothesis was that low-dose growth hormone increases accretion of muscle mass and attenuates bone mineral wasting when given daily during outpatient convalescence, from hospital discharge through 1 year after severe burn.
METHODS
Patients
Seventy-two severely burned children were enrolled in a 1-year trial investigating the effects of continuous growth hormone administration on muscle accretion and growth. Entrance criteria included age younger than 18 years, TBSA burns of more than 40%, acute burn treatment at Shriners Burns Hospital-Galveston, and consent to return at 6, 9, and 12 months after burn. This study was performed under a protocol approved by the University of Texas Medical Branch Institutional Review Board. Informed written consent was obtained from each patient’s guardian with the assent of the child before enrollment.
Patients underwent indirect calorimetry measurements 1 week after hospital admission to treat their acute burns. This and further measurements of indirect calorimetry were performed between midnight and 5 am while the patient was asleep.
On the day before discharge, patients entered the study protocol (Fig. 1). Standing height and nude weight were recorded using a standardized length device and a sling scale. Dual-energy x-ray absorptiometry (DEXA) was performed to measure lean body mass and bone mineral content. On the morning of discharge, blood was drawn at 7 am to determine growth hormone, insulinlike growth factor (IGF) 1 and IGF-binding protein (IGFBP) 4 and 5, insulin, parathormone, and osteocalcin levels. These metabolic and body composition studies were repeated at 6, 9, and 12 months after burn.

Figure 1. Study procedures during the year after burn. REE, resting energy expenditure; DEXA, dual-energy x-ray absorptiometry.
On discharge from the intensive care unit, patients began daily subcutaneous drug or saline placebo injections. Family members were taught to administer the injections in all cases, and patients did not leave Galveston until proficiency and reliability were shown. Growth hormone patients were given a 3-month supply of recombinant human growth hormone (rhGH) (Lilly, Indianapolis, IN). A dosage of 0.05 mg rhGH/kg per day was chosen based on demonstrated efficacy during long-term treatment of children with Turner syndrome. 14 Placebo patients were supplied with an equal amount of saline vehicle. The hospital research pharmacist performed randomization and initiation of pharmacotherapy. Patients and clinicians were unaware of therapy.
Seven of the 19 children in the growth hormone group and 8 of the 21 in the placebo group participated in an in-hospital physical rehabilitation program between 6 and 9 months after injury. Families of all other children were given standard range-of-motion physical therapy exercises, which patients were instructed to perform daily. Physical and occupational therapy referrals to departments close to the children’s homes were given for clinically identified problems.
Seventy-two children were initially enrolled in the study. Ten could not return at all proscribed measurement time points (because of family, school, or other travel constraints). During a 2-month period, 13 children were unable to undergo DEXA scanning despite appropriate follow-up for technical difficulties. Six subjects refused injections. Three subjects (all 3 years of age or younger) were unable to tolerate the short-term immobility required for DEXA scanning. In all, 40 children completed all measurements at the 6-, 9-, and 12-month postinjury time points.
Measurement of serum hormone levels was initiated approximately midway through this clinical trial. Twenty-one children had hormone levels measured at 6, 9, and 12 months after injury.
Body Composition
Total body lean mass and bone mineral content were measured by DEXA. A Hologic model QDR-1000W DEXA (Hologic Inc., Waltham, MA) was used to measure body composition for most of the study but was replaced when nonfunctional by a QDR-4500A Absorptiometer (Hologic Inc.). To minimize systematic deviations, the Hologic system was calibrated daily against a spinal phantom in the anteroposterior, lateral, and single-beam modes. Individual pixels were calibrated against a tissue bar phantom to determine whether the pixel was reading bone, fat, lean tissue, or air. Plain anteroposterior and lateral tibia–fibula x-rays were taken of each child at each follow-up period to evaluate possible premature closure of epiphyseal plates induced by anabolic agents.
Hormone Panel
Five milliliters of whole blood was withdrawn from an indwelling central venous line for determination of hormone levels. All levels were measured using enzyme-linked immunosorbent assays purchased from Diagnostic Systems Laboratory (Webster, TX). IGFBP-4 and -5 levels were measured by radioimmunoassay as previously described by Klein et al 15 on available sera.
Indirect Calorimetry
Resting energy expenditure was measured using a Sensor-Medics 2900 metabolic cart (Yorba Linda, CA). Inspired and expired gases were sampled and analyzed at 60-second intervals. Values of Vco2, Vo2, and resting energy expenditure were accepted during a 5-minute steady state. The average resting energy expenditure was calculated from these steady-state measurements. All indirect calorimetry measurements were made at 30°C, the standard environmental setting for all patient rooms in our acute burn intensive care unit.
Statistical Analysis
Data are presented as means ± standard error of the mean or means ± 95% confidence intervals where appropriate. Paired and unpaired t tests were used to compare interval data when indicated. Proportional comparisons between groups were done using a z test. P < .05 was considered statistically significant. Statistical software (SigmaStat and SigmaPlot; SPSS, Chicago, IL) was used to perform all analyses.
RESULTS
From June 1998 to December 1999, 40 severely burned children completed this study. Nineteen children and their families had been supplied with growth hormone and 21 had received saline placebo. Demographics of the groups are shown in Table 1. In general, these were young children with massive burns. No significant differences in terms of age, sex, or burn size were found between groups.
Table 1. DEMOGRAPHICS
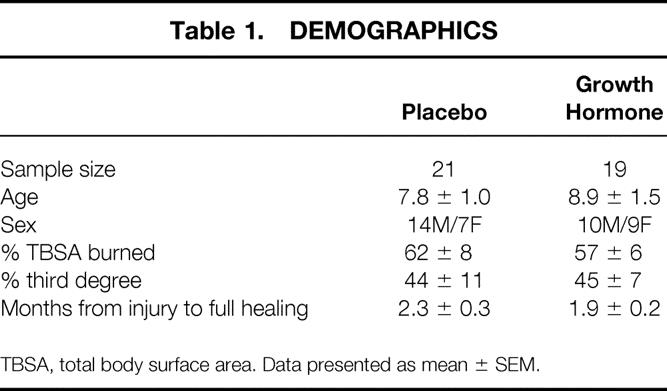
TBSA, total body surface area. Data presented as mean ± SEM.
Changes in total body weight and linear height during the 12-month postburn study period are depicted in Table 2. Children receiving growth hormone gained more weight than placebo subjects at the 9-month study point (P < .05). This disparity in weight gain continued to expand throughout the remainder of the study period. An increase in linear growth (standing height) was also seen in the growth hormone group (P < .05). Children receiving growth hormone grew approximately fivefold more rapidly than placebo subjects during the course of this study.
Table 2. CHANGES IN STANDING HEIGHT AND NUDE WEIGHT FROM BASELINE AT DISCHARGE

rhGH, recombinant human growth hormone. Data presented as mean ± SEM.
*P < .05 by unpaired t test.
Changes in body composition are shown in Figures 2 and 3. Accretion of lean body mass was accelerated by growth hormone treatment. Differences between the groups were significant after the first 3 months of therapy and continued to increase throughout the entire study period (P < .05). Lean body mass of placebo subjects did not statistically change from baseline during the entire 12-month period from the baseline measured at full wound healing.

Figure 2. Lean body mass changes measured by serial dual-energy x-ray absorptiometry (DEXA) scans in the same children.

Figure 3. Bone mineral content changes in serial dual-energy x-ray absorptiometry (DEXA) scans from the same children.
Whole body bone mineral content markedly increased in the children receiving growth hormone. Bone wasting, as indicated by diminishing bone mineral content, was apparent in both groups between discharge and 6 months after injury (P < .05 for both groups from zero). Placebo subjects had no further change in bone mineral content, whereas the children receiving growth hormone accrued bone mass between 6 and 9 months; this continued throughout the remainder of the study. These findings parallel the changes in standing height presented in Table 2. No changes were found in bone mineral density, indicating that growth hormone treatment increased both bone mineral content and bone area compared with placebo.
Children in both groups were hypermetabolic relative to the basal metabolic rate predicted by the Harris-Benedict equation (Fig. 4). Interestingly, systemic energy expenditure was increased in the children receiving growth hormone (P < .05 at 12 months by unpaired t test) compared with placebo subjects.

Figure 4. Resting energy expenditure as a percentage of the predicted basal metabolic rate (BMR) over time in burned children treated with growth hormone or placebo.
Twenty-one children (12 in the growth hormone group and 9 in the placebo group) had serum hormone profiles measured at all follow-up periods; these were used for further analysis. As expected, daily rhGH administration resulted in higher serum growth hormone levels than placebo (P < .05 by unpaired t test at 6 and 9 months) (Table 3). IGF-1 and IGFBP-4 and -5 levels were not different between groups, but IGFBP-4 levels remained persistently elevated. Levels of parathormone were very low in both treatment groups, but a significantly higher percentage of children receiving growth hormone had levels that were at least detectable by enzyme-linked immunosorbent assay (P < .05 by z-test at 12 months) (Fig. 5). Other hormone values were not different between groups. Serum levels of osteocalcin, the marker of bone formation, were low despite growth hormone therapy.
Table 3. HORMONE LEVELS
rhGH, recombinant human growth hormone; IGF, insulinlike growth factor; IGFBP, insulinlike growth factor-binding protein.
Data presented as mean ± SEM.
*P < 0.05 by unpaired t test.
†P < .05 by z test.
Number samples were different for IGFBP-4 and -5 because of lack of serum for these analyses from the original samples.
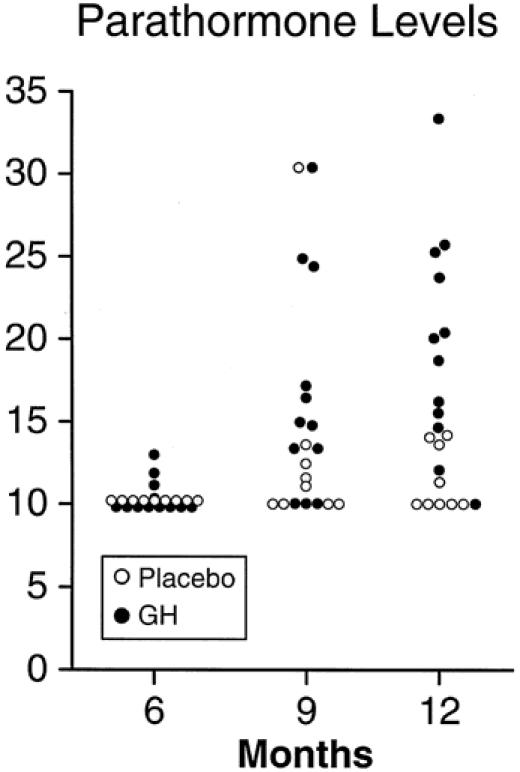
Figure 5. Scatter plot of parahormone levels with and without growth hormone (GH) treatment in severely burned children.
No children were hyperglycemic during follow-up. None experienced development of hirsutism or precocious sexual development. No child receiving growth hormone had premature closure of long bone epiphyses.
DISCUSSION
With wide acceptance of early definitive surgical treatment of burns and improved nutrition and other critical care support, the death rate after severe burn has drastically decreased in the past 30 years. 16 As more severely burned patients survive to become rehabilitated and reenter society, long-term complications of the hypermetabolic response have become increasingly apparent. We recently determined that muscle catabolism persists for at least 7 months after full healing of all wounds in children burned over 40% TBSA. 3 In the current study, we continued our investigation of the metabolic complications of burn in the first year after injury. Here we show that the prolonged metabolic disturbance after severe injury may be treated successfully with anabolic pharmacotherapy. The daily administration of growth hormone given to severely burned children subcutaneously at a dosage of 0.05 mg/kg after hospital discharge for 1 year increased total body weight, linear growth, lean body mass, and bone mineral content compared with an equivalent group receiving placebo.
In this randomized, double-blind clinical trial, 56% (40/72) of accrued subjects completed all evaluation points during the 12-month study. Thirteen of the 32 children did not complete all aspects of the trial for technical reasons (DEXA scanner malfunction), giving an overall 73% completion rate. The number of children completing the trial was similar in each group, and the reasons for dropout were similarly distributed for both groups. Therefore, we believe that no bias has been interjected into the study to cause the differences seen between groups.
Hormone enzyme-linked immunosorbent assays indicated that a daily subcutaneous injection of rhGH at a dosage of 0.05 mg/kg was successful at increasing systemic growth hormone levels. At this dosage, no child was found to be hyperglycemic at any follow-up period. No other potential complications of anabolic therapy were noted (e.g., hirsutism, precocious sexual development, epiphyseal closure).
The anabolic effects of growth hormone are thought to mediated, at least in part, through IGF-1. We found no differences in IGF-1 levels or IGFBP levels between the groups. These data are not powerful enough to reject this proposed effector mechanism, however. Either insensitivity of enzyme-linked immunosorbent assays or simple type 2 error may be responsible for the lack of statistically significant differences. Also of interest, however, is that IGFBP-4 levels remained elevated up to 1 year after injury, despite the anabolic changes to bone and muscle secondary to growth hormone treatment. This suggests that IGFBP-4 has limited clinical significance by itself in blocking the anabolic effects of growth hormone treatment in burn patients.
Standing height and gross body weight were dramatically increased in the children receiving growth hormone. The children receiving placebo essentially did not grow for a year after injury. This is consistent with previously reported data from our institution. 4,17 However, with daily, low-dose growth hormone treatment, weight gain increased by 9 months and linear growth increased by 12 months after injury. These measures are relatively gross measures of anabolism in children, and the finding of changes in them highlights the effectiveness of growth hormone in this setting.
These gross clinical measures of anabolism are corroborated by DEXA determination of lean body mass and bone mineral content. As shown in Figure 3, accretion of muscle mass was accelerated in the children receiving growth hormone. This effect was evident by the first evaluation period (6 months after injury) and continually increased throughout the remainder of the study. Whole body bone mineral content in both groups was below the discharge baseline at 6 months after injury. In the placebo group, osteopenia persisted during the entire 12-month study period. Administration of growth hormone abolished bone mineral wasting by 9 months after injury. Net bone formation accelerated through the remainder of the study period.
Lean body mass changes were evident before significant changes in bone mineral content were found. It is known that bone mineral content changes in response to muscle loading in several patient populations. We speculate that the bony response to growth hormone treatment may be indirect, in part a result of greater muscle strength and thus more bone loading. Further post hoc subanalysis revealed no differing responses in terms of burn size or sex among either group.
Parathormone levels were elevated in the children receiving growth hormone. Growth hormone therapy has not previously been reported to alter endogenous parathormone levels in burn or other growth hormone-deficient patients. It is not clear whether this represents a signal mediator mechanism of growth hormone or a secondary elevation in response to accelerated bone mineral turnover affected by some other growth hormone-mediated pathway. Further study of the mechanics of calcium metabolism after burn is warranted.
Energy expenditure was increased in the children receiving growth hormone at the 12-month time point. We have not observed this in other clinical studies involving growth hormone, but none of these previous trials consisted of therapy of several months’ duration. A potential explanation for this finding lies in the anabolic effects of growth hormone. Among other effects, it is known to stimulate muscle protein synthesis, which requires energy. 18 Skeletal muscle contributes approximately 30% of the basal metabolic rate in severely burned patients, 19 so it is not surprising that patients with greater lean mass would have greater energy expenditures. It may therefore be that elevated energy expenditure for the first year after severe burn is a byproduct of improved muscle protein kinetics. Studies with other anabolic agents would be required to test this assertion.
Although stimulation of linear growth and muscle protein synthesis are well-known effects of short-term growth hormone therapy, this is the first time that effects of growth hormone on clinically relevant outcomes have been measured through long-term rehabilitation. As evidenced by our placebo group, catabolism of muscle mass, bone mineral wasting, and growth retardation are profound after severe trauma such as burn. Growth hormone therapy improved these metabolic disturbances measured by gross clinical parameters (height and weight) and by scientific determination of lean body mass and bone mineral content. Our results also show that growth hormone administered at a dosage of 0.05 mg/kg per day is safe and efficacious. Left to be determined are the questions of safety and efficacy with escalating dosages. Larger dosages (up to 0.2 mg/kg per day) have shown proportionally greater gains in growth and wound healing, but they have also shown an increased frequency of hyperglycemia. 8,9 Further study is necessary to determine the optimal balance of safety and efficacy.
Although improved muscle mass and accelerated linear growth are not identical to improved rehabilitation efforts or hastened productivity in society, they are concrete outcome measures that may be readily extrapolated to real rehabilitation potential. Certainly, body composition studies have limitations. Particularly germane to burns is the confounding influence of cellular and whole body water, on which many of the DEXA modeling calculations are based. To avoid this as a potential criticism, we initiated treatment and evaluation when patients’ wounds were fully healed and subjects were discharged from the intensive care unit. At this point, body composition was stable and these burn patients were not undergoing fluid shifts characteristic of resuscitation or excisional therapy.
In summary, treatment with low-dose rhGH successfully abates the muscle catabolism and osteopenia induced by severe burn. Daily administration of 0.05 mg/kg is safe and efficacious as outpatient anabolic therapy. Efficacy is retained throughout several months of treatment. Future studies examining escalating dosages of growth hormone as well as less noxious anabolic pharmacotherapy (e.g., the oral testosterone analog oxandrolone) are indicated.
Discussion
DR. BASIL A. PRUITT, JR. (San Antonio, Texas): Drs. Hart, Wolf, and Herndon have provided additional data on the utility of growth hormone administration in pediatric burn patients, building upon their recent description of the prolonged hypermetabolic and catabolic response which may last up to 1 year. Growth hormone, when administered in the rehabilitative phase, appears to restore the previously documented growth retardation which accompanies severe pediatric thermal trauma. The strengths of this project include its design and the prolonged follow-up.
I have several questions concerning the conduct of the study. Did all patients receive anabolic agents during the acute phase? How was diet and exercise controlled during the rehabilitative phase? Were there differences in the regimen for reconstructive surgery between the two groups?
Concerning the mechanism of effect, why do you suppose you could not detect an effect on IGF-1 levels? Did you measure binding protein levels as well?
Most important is whether the recollections have any clinical relevance. Do you have any measures of strength indicating a functional improvement? Did exercise tolerance increase?
Finally, although there was an increase in growth in the treated group, how did this compare to unburned children? There must be significant functional benefit to this treatment.
Dr. Hart, Dr. Herndon and their colleagues have added another fine chapter to their encyclopedic work describing the metabolic response to injury and its treatment. Today they have confirmed their earlier finding that postinjury hypermetabolism extends for at least 1 year after injury, and they report that long-term, 1-year, administration of human growth hormone can accelerate restoration of lean body mass and bone mineral content in children who are convalescing from severe burns.
To place those findings in perspective, we need to assess the cost-benefit ratio of such treatment. For the numerator of that ratio we need to know the cost of a year’s supply of growth hormone, and for the denominator we need to know the physical function correlates of the changes in lean body mass and bone mineral content: i.e., can the growth hormone-treated children walk or run farther, lift more weight, or exert greater grip strength? Conversely, have the patients in the placebo group had more fractures because of a lower bone mineral content?
To help us evaluate your findings and conclusions, I have a few additional questions. Since metabolic rate is related to both body mass and food intake, is the differential increase in resting energy expenditure in the growth hormone-treated patients simply a manifestation of greater body mass and/or greater food intake? How did you assess comparability of food intake and physical exercise in the two groups?
The number of patients in both groups who participated in the in-hospital exercise program was comparable, but they were all supposed to carry out physical therapy exercises on a daily basis at home, and we need to know how compliance with that program was monitored and whether exercise was comparable between the two groups.
The only demographic variable that I can identify that was different was the male-to-female ratio in the two groups. In the placebo group, that was two to one, males to females. In the growth hormone group, that approached one to one. Is the effect of growth hormone greater in female children following burn treatment?
You have previously reported that the effect of growth hormone on bone growth is most evident during growth spurts. In light of that, did the growth hormone children simply have more growth spurts during the study period?
Is the growth hormone dose of 0.05 mg/kg/day the optimum dose? Or if you doubled the dose to 0.1 mg/kg/day, would the effect double and a 10-fold rather than a 5-fold increase in the growth rate occur in the growth hormone-treated patients as compared to the placebo-treated patients?
Lastly, I note that there was a 32% dropout rate, and I wonder how many dropped out because of dislike of repeated injections. Can that problem be eliminated by the use of the androgenic agent oxandrolone, which you have reported to have similar metabolic effects in convalescent burn patients and has the advantage of oral administration and lower cost?
Finally, I think it is important to put this report into perspective. As recently as 50 years ago, concern with these children would be that over half of them would have died. Today, because of the work of Dr. Herndon and other groups, the concern is with convalescence and quality of life.
I thank the Association for the privilege of the floor.
DR. WILLIAM C. CIOFFI, JR. (Providence, Rhode Island): Thank you, Dr. Aust, Dr. Townsend, fellows, and guests. Similar to Dr. Pruitt, I congratulate Dr. Hart and Dr. Herndon on providing additional data on the utility of growth hormone admission in pediatric burn patients, especially building upon their recent description of the prolonged hypermetabolic and catabolic response which lasts up to 1 year. They have concluded that growth hormone administered in the rehabilitative phase appears to restore the previously documented growth retardation which accompanies severe pediatric thermal trauma. I have several questions to help us analyze their data.
First, concerning the conduct of the study, and as Dr. Pruitt alluded to, were all patients treated similarly during the acute phase? Were anabolic agents administered to patients during their acute phase of care? And if so, was this similar between the two groups? Likewise, how was diet and exercise controlled for, especially for those patients who were not in the in-house exercise program? Was there a difference between requirement for reconstructive surgery between the two groups, with potentially the placebo group making more trips back to Galveston for surgery and the potential detrimental effects of multiple surgical procedures on growth, etc.?
Concerning the mechanism of effect, why do you suppose you could not detect an effect on IGF-1 level, since, presumably, the metabolic actions of growth hormone are mediated through this compound? Did you measure binding protein levels such as BP-3? And if so, were they increased, allowing increased bioavailability of IGF-1, although the levels of IGF-1 were not elevated?
And most importantly, as Dr. Pruitt alluded to, is there any clinical relevance to your findings? Although the treated patients had increased growth, do you have any measures of strength, etc., indicating any functional improvement? Did exercise tolerance increase?
And, finally, although there was an increase in growth in the treated groups, how did this compare to unburned counterparts? Because a small increase in growth may not be worth the added expense and pain of administering the drug if they still lag far behind unburned children.
My last comment relates to the increase in parathormone levels, etc. And I wonder if there were any increased complications associated with increased PTH in calcium in this group of patients.
In summary, this is a well-performed blinded randomized trial which helps put in perspective the care of these patients in the rehabilitative phase, and I think Dr. Herndon and his colleagues should be congratulated for focusing on this aspect of these patients’ care. Thank you.
DR. ANDREW M. MUNSTER (Baltimore, Maryland): Mr. President, Mr. Secretary, members, and guests. Little is left to ask after the previous two speakers, but I do have a couple of questions and a couple of comments.
Firstly, I trust you all realize what a difficult study this must have been, and I am amazed that the dropout rate was actually as low as it was. We are talking about daily injections into children. For the hormone measurements, we are talking about the outpatient placement of central venous lines for sampling, so this is a very, very difficult study, and I think they did very well. They are to be congratulated.
I would like to echo a couple of the questions as far as functional outcome measurements are concerned in these children, particularly in view of the fact that there are now a couple of well-documented instruments available to do that – one recently developed by the American Burn Association and the Shriners together, which is essentially a quality-of-life functional instrument – and I wonder if Dr. Herndon and Dr. Hart have thought of applying this tool to measuring what these children are truly like.
I also would like to see some comparison with normal children, because my children are too far grown to remember whether 3 inches a year is too much or normal, or 15-lb increase in weight in 1 year is too much or normal.
I was intrigued by the low osteocalcin levels. I wonder if Dr. Herndon could speculate what was the mechanism of the increase in bone and mineral content, given that there was no increase in osteocalcium levels.
And, finally, as an alternative to subcutaneous injections, would it be possible to administer this drug by pump, such as an insulin pump?
I thank the Association for the privilege of the floor and the authors for allowing me to see the manuscript.
DR. RANDALL POWELL (Mobile, Alabama): Dr. Herndon and his colleagues have added another significant segment in the continuing saga of the use of growth hormone to ameliorate the effects of large burn injuries in children. I basically have two questions.
One, what is the cost of the therapy? Recombinant growth hormone has typically been fairly expensive. And, two, is there any effect in the maturation of the burn wound? Specifically, is there any effect on hypertrophic scarring or contracture?
I’d like to thank the Association for the privilege to discuss this.
DR. DAVID N. HERNDON (Galveston, Texas): I’d like to thank all the discussants for their excellent comments.
Dr. Pruitt and Dr. Powell both and Dr. Cioffi asked about cost of treatment. This is an expensive treatment, and for a young child it would cost $3,500 for a year’s worth of treatment for 10 months, at least, from the time of discharge until the end of this study. It could cost as high as $5,000 per year for the mean child in this group, and if it were to be used on a 70-kg individual, the cost could be $9,000 per year.
The cost-benefit ratio is what was being addressed, and functional outcomes were called for by many of the discussants, and we will do that. We do not have sufficient numbers for the wide variability and various strength tests and functional outcome instruments to definitively answer the questions posed with this sample size, and a larger number of patients will be required to show changes in functional outcome over time.
Dr. Pruitt did properly point out that metabolic rate does change with body mass, and the small difference in those patients treated with growth hormone, metabolic rate, and placebos at the end of the year, likely is due to body mass.
There were questions about difference in exercise between the different groups throughout this treatment period and also questions about potential differences in diet in these groups. As patients were sent home, they were not strictly monitored for caloric intake and exercise except by questionnaires. The reliability of questionnaires for dietary intake and the reliability of questionnaires for compliance to exercise programs can truly be questioned. There were no differences between the groups using these instruments. They are not included in the description in the paper because of the subjective nature of those particular organs.
Dr. Pruitt mentioned a 32% dropout rate. Really, only six of the patients dropped out because they didn’t want to take injections, but the injections are very difficult to take. The 32% dropout refers to patients not completing all data points in this particular study, and we felt for statistical analysis we wanted to have a group in which every single measurement had been made. There were patients who dropped out because our DEXA machine broke for a period of time, and there were patients who could not return precisely at the time intervals that we asked for. But only six objected to the injections. Nonetheless, oxandrolone is a substance which can be given orally and is much less expensive than the $3,000 to $9,000 that would be required for use of growth hormone. And it should be tested, and we intend to do so in the future.
Dr. Cioffi asked if all patients were treated similarly during the acute phase in terms of anabolic agents. This study was conducted at a time when we were not giving growth hormone to acute patients. There was a study in Europe in which giving growth hormone during acute treatment of critically ill adults had an increase in mortality. We put a moratorium on the use of growth hormone during analysis of our large database to address whether there was any increased risk in treating patients acutely in the hospital with recombinant growth hormone. We found that there was no increased risk in the pediatric burn patient population. Nonetheless, during this study period, these patients were not receiving growth hormone during acute hospitalization.
They were treated in reverse crossover studies, studying agents such as propranolol, insulinlike growth factor 1 and BP-3, but all of them were crossover studies and each of the patients were treated, and there were no differences between treatment groups for this study.
There was no difference in the amount of exercise done, Dr. Pruitt, to those patients who were in-house. Compliance appeared to be the same for those that were outside of the house, but could not be absolutely determined.
Dr. Munster asked about the low osteocalcin levels. If it were not osteocalcin levels, what is the mechanism of improving bone formation? There are variations in IGFBP-4 levels and IGFBP-3 levels which may account for these things. The fact that the IGF-1 levels were not different, they tended to be different. I think a larger number of patients, they will be different. The ratio between IGFBP-3 and IGF-1 is critical in this, and I think simply a larger sample size will bear those things out.
Dr. Pruitt asked were there more fractures in the placebo group. Not in this small group, but in a large group of patients, we have shown an increased fracture risk in this patient population. We have shown a decrease in growth. The growth that we saw in this study with a dose of 0.05 mg/kg/day was normal, Dr. Munster and Dr. Pruitt. If we were to give more, 0.1 mg/kg/day, perhaps we could make growth greater than normal, but I’m not sure that that is desirable. We will, nonetheless, conduct a growth response curve to find out what the minimal dose is – that is, the least expensive and least dangerous dose available.
Dr. Powell asked what was the effect on scar. We have a large series of patients treated with high-dose recombinant human growth hormone during acute hospital stay, in which we followed scar formation for 2 years to 4 years postinjury and showed no effect of acute administration of growth hormone on scar maturation. This group is too young, after burn that is, to determine whether that effect will be similar. But I suspect that it will be similar and that there will be no differences in scar in this group.
I thank the Association for the opportunity to respond.
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Supported by the National Institutes of Health Grants #P01-GM60338-01, #R01-GM56687-04, and #T32-GM08256-07, National Institute for Disability and Rehabilitation Research Grant #H133A70019, and Shriners Hospital Grants #8660 and #8952. Recombinant human growth hormone was provided as a gift from the Eli Lilly Corp. (Indianapolis, IN).
Correspondence: David N. Herndon, MD, Shriners Hospital for Children, 815 Market St., Galveston, TX 77550.
E-mail: dherndon@utmb.edu
Accepted for publication December 2000.
References
- 1.Moore FD. Response to starvation and stress. In: Moore FD, ed. Metabolic care of the surgical patient. Philadelphia: WB Saunders; 1959: 202–275.
- 2.Warden GD, Heimbach DC. Burns. In: Schwartz SI, ed. Principles of surgery. New York: McGraw-Hill, Inc.; 1999: 232–254.
- 3.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery 2000; 128: 312–319. [DOI] [PubMed] [Google Scholar]
- 4.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg 1990; 125: 392–395. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson DP, Shaw GB, Young FG. The anterior pituitary gland and protein metabolism. II. The influence of anterior pituitary extract on the metabolic response of the rat to injury. J Endocrinol 1941; 2: 468–474. [Google Scholar]
- 6.Byrne TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg 1993; 218: 400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 8.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg 1990; 212: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilpin DA, Barrow RE, Rutan RL, et al. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994; 220: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarrar D, Wolf SE, Jeschke MG, et al. Growth hormone attenuates the acute-phase response to thermal injury. Arch Surg 1997; 132: 1171–1175. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med 2000; 28: 1578–1584. [DOI] [PubMed] [Google Scholar]
- 12.Knox J, Demling R, Wilmore D, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma 1995; 39: 526–530. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez RJ, Wolf SE, Barrow RE, et al. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 1998; 228: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rongen-Westerlaken C, Wit JM, De Muinck Keizer-Schrama SM, et al. Growth hormone treatment in Turner syndrome accelerates growth and skeletal maturation. Dutch Growth Hormone Working Group. Eur J Pediatr 1992; 151: 477–481. [DOI] [PubMed] [Google Scholar]
- 15.Klein GL, Wolf SE, Langman CB, et al. Effects of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab 1998; 83: 21–24. [DOI] [PubMed] [Google Scholar]
- 16.Wolf SE, Rose JK, Desai MH, et al. Mortality determinants in massive pediatric burns. An analysis of 103 children with ≥80% TBSA burns (≥70% full-thickness). Ann Surg 1997; 225: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study [letter]. Lancet 1999; 354: 1789. [DOI] [PubMed] [Google Scholar]
- 18.Yu YM, Tompkins RG, Ryan CM, et al. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. J Parenter Enteral Nutr 1999; 23: 160–168. [DOI] [PubMed] [Google Scholar]
- 19.Wilmore DW, Aulick LH. Systemic responses to injury and the healing wound. J Parenter Enteral Nutr 1980; 4: 147–151. [DOI] [PubMed] [Google Scholar]



