Abstract
Objective
To determine whether the evolution of the authors’ clinical pathway for the treatment of hemodynamically compromised patients with pelvic fractures was associated with improved patient outcome.
Summary Background Data
Hemodynamically compromised patients with pelvic fractures present a complex challenge. The multidisciplinary trauma team must control hemorrhage, restore hemodynamics, and rapidly identify and treat associated life-threatening injuries. The authors developed a clinical pathway consisting of five primary elements: immediate trauma attending surgeon’s presence in the emergency department, early simultaneous transfusion of blood and coagulation factors, prompt diagnosis and management of associated life-threatening injuries, stabilization of the pelvic girdle, and timely insinuation of pelvic angiography and embolization. The addition of two orthopedic pelvic fracture specialists led to a revision of the pathway, emphasizing immediate emergency department presence of the orthopedic trauma attending to provide joint decision making with the trauma surgeon, closing the pelvic volume in the emergency department, and using alternatives to traditional external fixation devices.
Methods
Using trauma registry and blood bank records, the authors identified pelvic fracture patients receiving blood transfusions in the emergency department. They analyzed patients treated before versus after the May 1998 revision of the clinical pathway.
Results
A higher proportion of patients in the late period had blood pressure less than 90 mmHg (52% vs. 35%). In the late period, diagnostic peritoneal lavage was phased out in favor of torso ultrasound as a primary triage tool, and pelvic binding and C-clamp application largely replaced traditional external fixation devices. The overall death rate decreased from 31% in the early period to 15% in the later period, as did the rate of deaths from exsanguination (9% to 1%), multiple organ failure (12% to 1%), and death within 24 hours (16% to 5%).
Conclusions
The evolution of a multidisciplinary clinical pathway, coordinating the resources of a level 1 trauma center and directed by joint decision making between trauma surgeons and orthopedic traumatologists, has resulted in improved patient survival. The primary benefits appear to be in reducing early deaths from exsanguination and late deaths from multiple organ failure.
Hemodynamically compromised patients with pelvic fractures present a complex challenge to trauma surgeons. Fractured pelvic bones bleed briskly and can lacerate surrounding soft tissues and disrupt their extensive arterial and venous networks. The resultant hemorrhage and secondary coagulopathy can be lethal; to confound matters, the considerable force required to fracture the pelvis typically results in significant associated extrapelvic injuries. Collectively, these factors account for high rates of death and complications. 1,2 Regional level 1 trauma centers, by their nature, are best equipped to manage these patients: experienced, dedicated trauma surgeons are immediately available, accompanied by a full complement of specialists including orthopedic traumatologists, interventional radiologists, and urologists 24 hours per day. Backed by the support of the operating room, surgical intensive care unit, and laboratory/blood banking services, the level 1 trauma team has the necessary tools for maximum patient survival. The key to optimizing patient outcome, then, lies in the decision making.
Fifteen years ago we emphasized our multispecialty approach to patients with pelvic fractures and significant hemorrhage;3 this concept has been subsequently reinforced by several groups. 1,2,4–6 Although the fundamental objectives—control of hemorrhage, restoration of hemodynamics, and prompt diagnosis and treatment of associated injuries—have not changed, the means of achieving these goals have evolved significantly. Maneuvers such as early mechanical pelvic stabilization 7–11 and arterial hemorrhage control by means of interventional radiologic techniques 12–14 are now recognized as pivotal components of the management scheme. During the past several years, we have matured a multidisciplinary clinical pathway for hemodynamically unstable patients with biomechanically unstable pelvic fractures that consists of five primary elements: immediate attending trauma surgeon’s presence in the emergency department to promote rapid, accurate decision making; early simultaneous transfusion of blood and coagulation factors to attenuate and potentially to preempt coagulopathy; prompt identification and management of associated life-threatening injuries; stabilization of the pelvic girdle; and timely insinuation of pelvic angiography and embolization. In May 1998, coincident with the addition of two orthopedic pelvic fracture specialists to our staff, we revised our pathway. The fundamental changes included immediate emergency department presence of the orthopedic trauma attending to provide joint decision making with the trauma surgeon; an emphasis on closing the pelvic volume in the emergency department by wrapping the pelvis with a sheet and taping the knees and ankles together; and the use of alternatives to traditional external fixation devices, such as antishock (“C”) clamps 15 or early surgical fixation. The purpose of this study was to determine whether the evolution of our multidisciplinary clinical pathway has improved patient outcome.
METHODS
Patients
Denver Health Medical Center, a state-certified and American College of Surgeons-verified urban level 1 trauma center with pediatric commitment, serves as the Rocky Mountain regional trauma center for Colorado and adjoining regions. Our prospective trauma registry identified patients with pelvic fractures, and blood bank records were cross-referenced to identify patients receiving blood transfusions in the emergency department.
Clinical Pathway
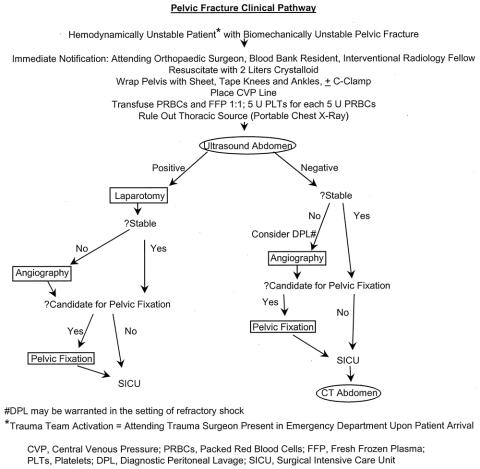
The first iteration of our multidisciplinary clinical pathway was implemented in late 1993, and it was revised in May 1998. The modification coincided with the addition of two orthopedic pelvic trauma specialists (W.R.S., S.J.M.) to our staff. Patients are resuscitated in concert with the guidelines of the American College of Surgeons Committee on Trauma Advanced Trauma Life Support. 16 In brief, hemodynamically unstable patients are orotracheally intubated and intravenous access is secured with at least two large-bore catheters. Central venous pressure monitoring is initiated along with crystalloid resuscitation. In conjunction with this, an ultrasound examination of the torso is performed to detect hemoperitoneum and hemopericardium. An anteroposterior chest radiograph is obtained to exclude intrathoracic injury. Clinical examination and anteroposterior pelvic radiography are used to determine whether the patient has a biomechanically unstable pelvic fracture; if confirmed, the full clinical pathway is initiated (Fig. 1). The attending trauma surgeon is in the emergency department when the patient arrives, based on a generic trauma activation policy. The attending orthopedic surgeon is additionally summoned to the emergency department to work with the attending trauma surgeon on treatment prioritization. The pelvic volume is closed by securing sheets tightly around the pelvis and by taping the knees and ankles together. The interventional radiologist and blood bank representative (a dedicated on-call pathologist) are notified and mobilized. Transfusion of packed red blood cells and fresh-frozen plasma is initiated in a 1:1 ratio, and for every 5 units of packed red cells, 5 units of platelets are administered.
Figure 1. Pelvic fracture clinical pathway.
In the presence of significant hemoperitoneum on ultrasound examination (fluid stripe ≥1 cm, or fluid in two or more spaces), the patient is transferred to the operating room for abdominal exploration and treatment of associated injuries. During surgery, the pelvis is packed with laparotomy pads. If the patient’s hemodynamic status stabilizes, a determination is made with the orthopedic surgeon regarding the need for mechanical fixation of the pelvis. This may involve anterior plating of the pubic symphysis, or the application of an external fixation device. Posterior fixation (either surgical or computed tomography-guided percutaneous fixation 17) is usually deferred until a later time. If the patient remains unstable, a damage-control laparotomy is performed. 18,19 Laparotomy pads are left in place and the skin is closed with a running 2-0 nylon suture. The patient is transported to the interventional radiology suite, with the anesthesia and the trauma surgery/critical care team present for continued monitoring and resuscitation. Pelvic arteriography is performed, with therapeutic embolization as needed. After arteriography, the patient is returned to the operating room for a second-look damage-control laparotomy and repacked as necessary. If the patient’s physiologic status is favorable, surgical pelvic fixation may be performed at this time. A temporary abdominal closure (skin only or Bogota bag) is used at this stage to prevent the abdominal compartment syndrome. 18–20
If the initial ultrasound scan does not reveal fluid (diagnostic peritoneal lavage is performed if the results of the ultrasound examination are equivocal) but the patient remains hemodynamically unstable or requires ongoing blood transfusion despite mechanical pelvic stabilization, pelvic arteriography is performed immediately to search for and embolize arterial bleeding. If the patient is a candidate for pelvic fixation, it is performed after angiography. Otherwise, the patient is transported to the surgical intensive care unit for continued resuscitation and advanced monitoring.
Data Analysis
Data were managed with Microsoft Excel v. 7.0 software (Microsoft, Redmond, WA). Statistical analysis was performed on an IBM-compatible personal computer using StatMost 32 for Windows 95 (DataMost Corp., Sandy, UT) and SPSS 9.0 for Windows (SPSS, Inc., Chicago, IL). Continuous data are expressed as mean ± the standard error of the mean and were compared using analysis of variance with Scheffe post hoc analysis. Categorical data were compared using chi-square analysis or the Fisher exact test, where appropriate.
RESULTS
Patients
From January 1994 through December 1999, 19,941 patients were admitted to the hospital or to the emergency department observation unit of our institution after blunt trauma. Seven hundred thirty-two (4%) had pelvic fractures; of these, 216 (30%) required blood transfusions in the emergency department and are the focus of this report. Our clinical pathway was revised in May 1998. For this analysis, the patients were divided into two groups: before (early) and after (late) the May 1998 revision.
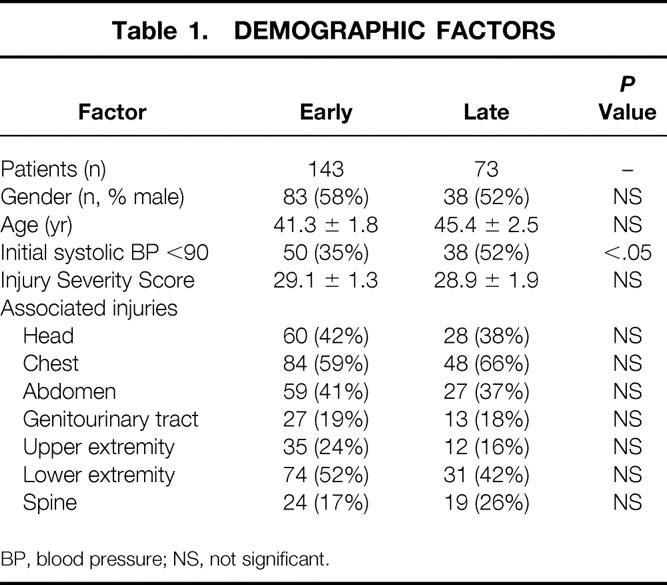
There were 143 patients treated in the early period and 73 in the late period. A greater percentage of patients in the late group had a systolic blood pressure of less than 90 mmHg (52% vs. 35%); otherwise, no differences existed in the age, gender distribution, injury severity score, or spectrum of associated injuries between the two groups (Table 1).
Table 1. DEMOGRAPHIC FACTORS
BP, blood pressure; NS, not significant.
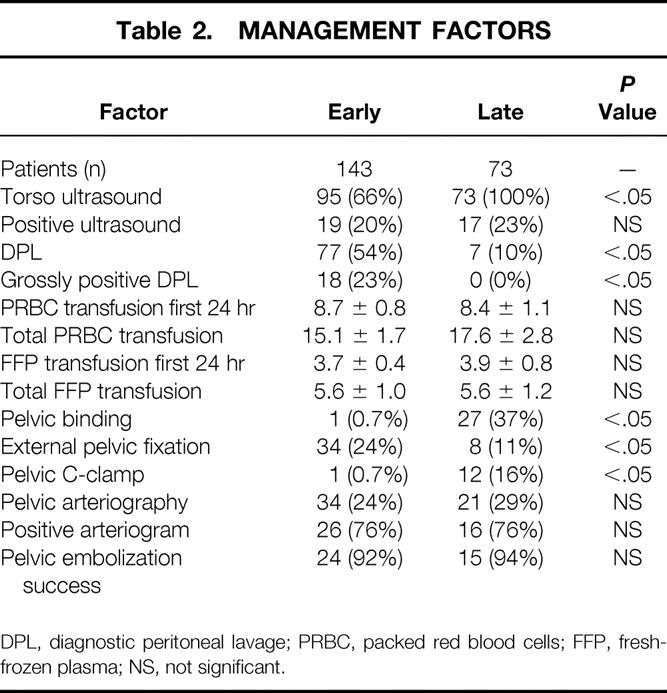
Torso ultrasound was being phased in during the early period, and thus confirmatory diagnostic peritoneal lavage occasionally followed a positive ultrasound result. In the late period, ultrasound was used uniformly and diagnostic peritoneal lavage was performed infrequently, generally in cases of equivocal results on ultrasound studies (Table 2). The incidence of either grossly positive diagnostic peritoneal lavage or a positive ultrasound scan in the early period (22%) was similar to that of positive ultrasound examinations in the late period (23%), indicating a stable incidence of significant hemoperitoneum leading to laparotomy. In the few patients undergoing diagnostic peritoneal lavage in the late period, there were no positive results, attesting to the accuracy of ultrasound. There were no differences in the number of packed red cells or fresh-frozen plasma units transfused. An emphasis on early pelvic binding with sheets and tape was evident in the late period. The use of the external pelvic fixator decreased in the late period, whereas the C-clamp device was applied more commonly; overall, mechanical stabilization devices were applied with similar frequency (24% in the early period, 25% in the late). Arteriography was used in 29% in the late period and 24% in the early period. In both periods, the rate of arterial bleeding requiring embolization was 76%, and the interventional radiologists were successful in definitively controlling the arterial hemorrhage greater than 90% of the time.
Table 2. MANAGEMENT FACTORS
DPL, diagnostic peritoneal lavage; PRBC, packed red blood cells; FFP, fresh-frozen plasma; NS, not significant.
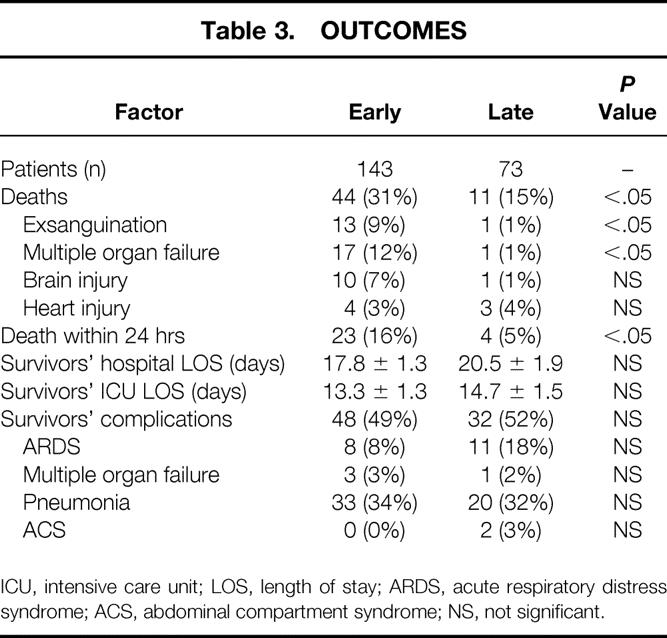
Outcomes are summarized in Table 3. There was a higher death rate in the early group (31% vs. 15%), as well as a greater death rate within the first 24 hours (16% vs. 5%) compared with the late group. The percentage of deaths from exsanguination decreased from 9% in the early group to 1% in the late group, and the death rate from multiple organ failure decreased from 12% to 1%. The hospital and surgical intensive care unit length of stay of survivors did not differ between the two periods. There were no significant differences between the two groups in terms of the overall complication rate or the occurrence of acute respiratory distress syndrome, multiple organ failure, or pneumonia. The occurrence of abdominal compartment syndrome was relatively low in both groups; overall, it was 1%.
Table 3. OUTCOMES
ICU, intensive care unit; LOS, length of stay; ARDS, acute respiratory distress syndrome; ACS, abdominal compartment syndrome; NS, not significant.
Available pelvic imaging studies were assessed in a masked fashion by the orthopedic traumatologists (W.R.S., S.J.M.), and pelvic fractures were classified according to the Young modification of the Pennal system. 21 There was no statistical difference in the Injury Severity Score, hypotension on admission, hemoperitoneum or genitourinary tract injuries, the use of pelvic angioembolization, or death among the injury classes. Combined mechanical injuries were associated with greater transfusion requirements.
DISCUSSION
As our clinical pathway has evolved, we have found it to be associated with an improved death rate. We believe that the presence in the emergency department of orthopedic traumatologists with a special interest in pelvic fractures has been critical to optimizing patient outcomes. Their knowledge and experience in the management of various fractures has allowed immediate, definitive decisions to be made early. Prompt closure of the pelvic volume appears to be an important maneuver in arresting hemorrhage, and early mechanical pelvic stabilization has been found to improve rates of death and complications and functional outcomes. 7–11 Meighan et al 22 noted that only 8 of 31 “major accident units” in Scotland could potentially stabilize a pelvis in less than an hour. Although no similar assessments have been made in level 1 and 2 trauma centers in the United States, we suspect wide variation exists.
Our practice has been initially to manage the hemodynamically compromised patient with a biomechanically unstable pelvic fracture by immediate reduction of the pelvic volume to promote tamponade of bleeding bone and vessels. Wrapping the pelvis with a sheet and binding the knees and ankles is a simple maneuver that can be performed by virtually anybody in the emergency department. We also recommend it to prehospital personnel and providers in rural hospitals to help stabilize patients for transport. These interventions can be used only temporarily, however, particularly when laparotomy is required. Long periods of tight immobilization of the abdomen or legs can contribute to abdominal or extremity compartment syndrome. Early in the course of resuscitation, a decision is made jointly between the attending trauma surgeon and the orthopedic trauma surgeon about the desirability of mechanical fixation, and which of several options is most appropriate. 23 Although the literature has suggested that early application of external frames is beneficial, 9,11,24 their placement can result in complications such as misplacement of pins, untimely delay in other necessary resuscitation maneuvers, and increased pelvic deformity. 25 Also, the pin tracts are often in line with incisions required for secondary reconstruction and may prevent appropriate definitive care, especially in the presence of pin tract infection. If fixation is deemed necessary and the injury pattern is amenable, a C-clamp is placed that can accompany the patient to the operating room, radiology suite, or surgical intensive care unit. The advantage of the C-clamp in our hands is that application takes less than 5 minutes and does not require fluoroscopy or other imaging. It can be placed in any setting (e.g., emergency department, operating room, radiology suite, surgical intensive care unit) and can be rotated cephalad or caudad to permit access to the chest, abdomen, pelvis, and extremities. In patients proceeding to the operating room for laparotomy, the clamp can be converted to an anterior plate at the same setting. We see very limited application for the pneumatic antishock garment, and it is not a part of our pathway.
Hemorrhage and secondary coagulopathy are well-recognized causes of death from pelvic fractures. We advocate aggressive crystalloid resuscitation, with immediate transfusion of packed red blood cells. It is our experience that coagulopathic bleeding becomes a clinical problem long before it is confirmed by coagulation tests. Consequently, we presumptively administer clotting factors (fresh-frozen plasma and platelets) immediately on identification of the pelvis as the primary source of hemorrhage.
One of the critical early decisions in the management of the trauma patient with a pelvic fracture who requires blood transfusions is to determine the source of blood loss. An overlooked torso injury may prove catastrophic if not controlled promptly; on the other hand, protracted diagnostic maneuvers intended to exclude such injuries could delay necessary interventions targeted at the pelvic hemorrhage, with resultant poor outcome. Our protocol emphasizes physical examination to look for external signs of hemorrhage, chest x-ray to exclude thoracic hemorrhage, and ultrasound to detect significant hemoperitoneum. The absence of alternative sites of hemorrhage allows one to presume the pelvis as the source. Moreover, prompt detection and treatment of extrapelvic bleeding sites may avoid unnecessary diagnostic evaluation of the pelvis (i.e., arteriography). As documented by many groups, 26–28 torso ultrasound is an accurate means of diagnosing hemoperitoneum. We have been successful in identifying significant hemoperitoneum with ultrasound, 26 and during the past several years have adopted it as our primary screening modality in the emergency department. Its application in unstable patients with pelvic fractures is clear because it allows rapid, noninvasive triage of patients. In general, if we find a fluid stripe more than 1 cm wide or expanding on serial examinations, or free fluid in two areas, we transfer the patient to the operating room. We have confirmed the accuracy of this approach: no patient in this series had a positive diagnostic peritoneal lavage or required laparotomy for bleeding in the presence of a negative ultrasound scan. Conversely, by requiring more than a thin fluid stripe to trigger laparotomy, we may minimize the number of nontherapeutic laparotomies.
We use the Young modification of the Pennal classification system 21 to standardize our communication regarding pelvic fractures. Although Burgess et al 29 reported different degrees of hemorrhage associated with the various fracture types, it has been our experience that patients with all types of fracture may be in shock, require blood transfusions, and challenge the trauma team to determine which injuries are life-threatening. Indeed, Cryer et al 30 attempted to identify patients at high risk, but their analysis showed that patients with any type of fracture are subject to hemorrhage from either the fracture or associated injuries. This has been the experience of Hamill et al 31 as well. Thus, we believe that the trauma team must be prepared to use all available modalities in any patient who is hemodynamically compromised, regardless of the apparent fracture geography. In fact, we chose to include all transfused patients in this analysis, because limiting it to subgroups (e.g., those with certain fracture types, or only those with initial hemodynamic instability) would ignore a large group of patients with complex management issues.
Arterial hemorrhage control by means of interventional radiologic techniques has proven to be a valuable adjunct to pelvic stabilization and transfusion. 12–14 Although most pelvic fracture-associated bleeding originates from bony edges and pelvic veins, arteriography is pivotal in controlling ongoing arterial bleeding in the pelvis. Although some groups use arteriography more liberally, based on fracture patterns or other parameters, we have found that the patients who do not recover with mechanical pelvic stabilization, transfusion, and treatment of associated injuries have a high likelihood (76%) of harboring pelvic arterial hemorrhage. Thus, we can limit our resource utilization without compromising patient well-being.
Abdominal compartment syndrome, a known complication of intraabdominal trauma, can result from retroperitoneal hematoma associated with pelvic fractures. Our experience 32 is consistent with that of the Memphis 33 and Houston 34 groups, that massive resuscitation in and of itself can lead to a secondary abdominal compartment syndrome. For that reason, we routinely leave the fascia open in pelvic fracture patients requiring exploratory laparotomy. 20 Further, our standard of care is to monitor bladder pressures every 2 hours in multitrauma patients in the surgical intensive care unit during the first 48 hours after injury to detect subclinical abdominal compartment syndrome.
In sum, the death rate among our patients has been cut in half during the late period of the clinical pathway. We believe this reflects a more effective multidisciplinary pathway. We advocate the following: field triage (urban) or interhospital transfer (rural) of critically injured patients to regional level 1 trauma centers; mobilization of a specialized trauma team from the outset, with joint emergency department presence of attending trauma and orthopedic surgeons; early aggressive resuscitation of these patients, with the administration of clotting factors along with packed red blood cells; rapid identification and treatment of associated injuries, with torso ultrasound as the primary screening modality; early stabilization of the pelvic ring; and timely and appropriate insinuation of arteriography in the management scheme.
Discussion
DR. TIMOTHY C. FABIAN (Memphis, Tennessee): I compliment the authors on documenting a large experience with significant pelvic fractures. They suggest that by defining a key clinical pathway they have improved their outcomes. While I believe their contention, there remains a fair amount of gray relative to the supporting data. This revolves mainly around attributable mortality. The authors compare two time frames for analysis, with the major difference being that in the late period, two orthopedic fracture specialists were added to their staff, resulting in a more aggressive approach to pelvic stabilization with pelvic binding and application of C-clamps and a lower reliance on other external fixators.
The early period encompassed 75% (4.5 years) of the 6-year study, while the late period was 25% (1.5 years); 66% of the patients were early and 34% were late. It may have been beneficial to analyze the results by quartiles to perhaps glean some idea of the potential impact of other variables beyond pelvic stabilization, such as transfusion policies and diagnostic approaches, including DPL, ultrasound, and CT. With those background comments, I would like to ask the following questions:
The early and late mortality rates were significantly different at 31% versus 15%, that at 24 hours was 21% and 4%, which leads to a virtually identical mortality at greater than 24 hours of 10% and 11%. If the impact of pelvic stabilization techniques accounted for the mortality reduction, one would expect less blood loss in the late group. Yet the transfusion requirements of red cells and components were virtually identical. How do you explain these somewhat disparate findings?
Similarly, one would think survivors’ complications would be higher in the early group due to less adequately treated shock, but in fact the complications were not different. What was the laparotomy rate in the two groups? Since DPL was commonly used in the early period, one might anticipate more laparotomies, which might have led to a negative impact on outcome.
In attempting to further evaluate differences in mortality between the two periods, could it be contributed to by closed head injury? Although similar rates of head injury are reported, this was made a dichotomous variable. Did you evaluate Glasgow coma scores between the groups? It is very possible that a few very bad head injuries could indeed partially account for the higher observed early mortality rate in the early group. An in-depth analysis of attributable mortality might aid in a clearer understanding of these issues.
Finally, the entry criteria for this study were unstable pelvic fractures and ED transfusion. These patients were multiply injured and had several sources for blood loss and requirement for transfusion. As I noted earlier, 34% of the population were entered into 25% of the study interval, the late period. Is it possible that transfusion policies were more aggressive with the addition of the orthopedic surgeons focusing on this problem, consequently including less critically injured? Addressing the study in quartiles and assessing denominator, i.e., total blunt admissions, may be some significant benefit in this analysis.
I very much enjoyed this paper and greatly appreciate the innovative leadership provided by Dr. Biffl and his colleagues at Denver in this and many other areas. As was the case last year when Dr. Biffl presented at the Southern their experience with blunt cerebral vascular injuries, we followed their lead. And I believe following this experience reported today, we will follow the same ideas in Memphis with pelvic fractures. Thank you.
DR. J. DAVID RICHARDSON (Louisville, Kentucky): I think it is an excellent paper, and we certainly have used a very similar protocol for managing patients for several years now, and would include ultrasound and attempt to decrease pelvic volume as early as possible.
The concern I have, anytime that you get into protocol- or algorithm-driven processes for pelvic fractures, is how you separate out the group of patients who are going to stop bleeding no matter what you do and the group of patients who continue to bleed almost no matter what you do.
Because I think in almost every large series of pelvic fractures that have been reported, there are a group of patients that need about 4 to 6 units of blood and then stop, often with relatively little intervention. And that remains a concern that if you jump on them immediately that you may in fact, as Dr. Fabian suggested, include patients that are not as critically injured, and that that may in fact considerably lessen your mortality.
So in terms of activation of the protocol, since only 52% had arterial pressure less than 90, what activated it? Were all the rest of those truly unstable fractures that would have required that activation? And if a patient is not hemodynamically unstable, how do you proceed?
My second question has to do with this transfusion threshold, and I think that is a key element and worry a little bit about starting what I would call a “moment 1” transfusion protocol. And, again, I was a little unclear about how you proceeded along your algorithm.
In our institution, I would say that from time to time I have been concerned that we embolize patients unnecessarily and that we sometimes do orthopedic stabilization on patients unnecessarily or with a notion that we are accomplishing something that I suspect in fact we are not. And I am just curious as to how you factor in those things.
The third point has to do with mortality and the cause of that, that Dr. Fabian alluded to. And that is, how do you really know that it has been the change in your management protocol that in fact has fairly sharply decreased your overall mortality? Because while your overall mortality decreased a lot, your hemorrhage mortality was less but couldn’t account for all of that, and I was just curious what other factors you might think related to that. Thank you very much.
DR. WALTER L. BIFFL (Denver, Colorado): Thank you, President Aust, Dr. Townsend, members, and guests. I’d like to thank the discussants for their review of the manuscript and comments and questions.
The premise behind this manuscript was not to focus on patients with isolated pelvic fractures or isolated bleeding from the pelvis, but rather to present the overall group that presents the complex challenge in the emergency department. And that is, the patient that comes in hemodynamically unstable or with borderline stability that requires blood transfusion in the emergency department.
Our group in the basic science laboratory over the last 10 years has identified blood as a harmful substance, and so we are not liberal with transfusion of blood in the emergency department. We are relatively selective. So the question is, what is it about these two time periods that has made a difference in the outcome of these patients?
Now to enroll patients in this study, they required transfusion in the emergency department. And, as Dr. Richardson pointed out, only about half the patients were hemodynamically unstable. So what was the trigger? Well, as our hemodynamically unstable definition was a blood pressure less than 90, there were young patients with tachycardia and a blood pressure hovering around 100 whom the attending surgeon decided to start transfusing blood.
The next question is, what is the source of the bleeding? And everybody has an algorithm for this, and the dozens of papers that have been published in the last 10 years, including Dr. Flint’s and Dr. Poole’s, everybody has an algorithm and they all focus on the same things: control hemorrhage, restore hemodynamic stability, identify and treat associated injuries.
That is what we have been doing for the last 15 years, but suddenly things changed and the outcomes started getting better. And what has been the reason for that? We are using the same transfusion trigger. We showed, as Dr. Fabian pointed out, that blood transfusion in the first 24 hours has not changed. Well, how do we explain that?
It is interesting that the number of patients with a blood pressure less than 90 has been significantly higher in the late group, but yet they have not received any more blood. So the converse of Dr. Fabian’s contention is that we are actually more effective in stopping the bleeding and, in fact, giving less blood to a group that we might be expected to give more blood to.
Head injury as a confounding factor in the mortality was not significant. We had four deaths from head injury in the early group, which accounted for less than 10% of those deaths. And so if we exclude head injury deaths, the mortality improvement was from 29% to 15%, which is still significant. There were a similar number of laparotomies in each group and a similar number of abdominal-injured patients in each group.
So, again, the question becomes, what explains the mortality improvement? And our belief is that it is the presence of the trauma surgery attending and the orthopedic attending in the emergency department together that has made the difference. All of our transfusion triggers, the role of angiography, have stayed pretty much the same over the years, but now we have an experienced, dedicated orthopedic surgeon there with us to individualize the treatment. I know Dr. Richardson has pointed out unnecessary interventions, and it may be that the external fixators being put on 5 years ago were unnecessary at the discretion of the orthopedic resident. But now we have that attending telling us what to do next, walking with us to the operating room, deciding whether to plate the pubic symphysis, and deciding when to go to angiography with us. And we think that has made the major difference. And whether it is combined femur and pelvic bleeding or pelvic and abdominal bleeding, we have not dissected out in this study. But the fact is, our mortality is half of what it was a few years ago, and we are encouraged by that.
I’d like to thank the Association for the opportunity to present these data.
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Correspondence: Dr. Walter L. Biffl, Department of Surgery, Box 0206, Denver Health Medical Center, 777 Bannock St., Denver, CO 80204-4507.
E-mail: wbiffl@dhha.org
Accepted for publication December 2000.
References
- 1.Fox MA, Mangiante EC, Fabian TC, et al. Pelvic fractures: an analysis of factors affecting prehospital triage and patient outcome. South Med J 1990; 83: 785–788. [DOI] [PubMed] [Google Scholar]
- 2.Poole GV, Ward EF, Muakkassa FF, et al. Pelvic fracture from major blunt trauma: outcome is determined by associated injuries. Ann Surg 1991; 213: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno C, Moore EE, Rosenberger A, et al. Hemorrhage associated with major pelvic fracture: a multispecialty challenge. J Trauma 1986; 26: 987–994. [DOI] [PubMed] [Google Scholar]
- 4.Flint L, Babikian G, Anders M, et al. Definitive control of mortality from severe pelvic fracture. Ann Surg 1990; 211: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruen GS, Leit ME, Gruen RJ, et al. The acute management of hemodynamically unstable multiple trauma patients with pelvic ring fractures. J Trauma 1994; 36: 706–713. [DOI] [PubMed] [Google Scholar]
- 6.Bassam D, Cephas GA, Ferguson KA, et al. A protocol for the initial management of unstable pelvic fractures. Am Surg 1998; 64: 862–867. [PubMed] [Google Scholar]
- 7.Goldstein A, Phillips T, Sclafani SJA, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma 1986; 26: 325–330. [DOI] [PubMed] [Google Scholar]
- 8.Latenser BA, Gentillelo LM, Tarver AA, et al. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma 1991; 31: 28–31. [DOI] [PubMed] [Google Scholar]
- 9.Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma 1993; 35: 671–677. [DOI] [PubMed] [Google Scholar]
- 10.Heini PF, Witt J, Ganz R. The pelvic C-clamp for the emergency treatment of unstable pelvic ring injuries. A report on clinical experience of 30 cases. Injury 1996; 27 (Supp 1):SA38–45. [DOI] [PubMed] [Google Scholar]
- 11.Scalea TM, Boswell SA, Scott JD, et al. External fixation as a bridge to intramedullary nailing for patients with multiple injuries and with femur fractures: damage control orthopaedics. J Trauma 2000; 48: 613–623. [DOI] [PubMed] [Google Scholar]
- 12.Panetta T, Sclafani SJA, Goldstein AS, et al. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. J Trauma 1985; 25: 1021–1029. [PubMed] [Google Scholar]
- 13.Piotin M, Herbreteau D, Guichard JP, et al. Percutaneous transcatheter embolization in multiply injured patients with pelvic ring disruption associated with severe haemorrhage and coagulopathy. Injury 1995; 26: 677–680. [DOI] [PubMed] [Google Scholar]
- 14.Agolini SF, Shah K, Jaffe J, et al. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma 1997; 43: 395–399. [DOI] [PubMed] [Google Scholar]
- 15.Ganz R, Krushell R, Jakob R, et al. The antishock pelvic clamp. Clin Orthop 1991; 267: 71–78. [PubMed] [Google Scholar]
- 16.American College of Surgeons Committee on Trauma. Advanced trauma life support, 6th ed. 1997.
- 17.Shuler TE, Boone DC, Gruen GS, et al. Percutaneous iliosacral screw fixation: early treatment for unstable posterior pelvic disruptions. J Trauma 1995; 38: 453–457. [DOI] [PubMed] [Google Scholar]
- 18.Burch JM, Ortiz VB, Richardson RJ, et al. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg 1992; 215: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore EE, Burch JM, Franciose RJ, et al. Staged physiologic restoration and damage control surgery. World J Surg 1998; 22: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 20.Offner PJ, de Souza AL, Moore EE, et al. Abdominal compartment syndrome is an avoidable complication of damage control laparotomy following trauma. Arch Surg (in press). [DOI] [PubMed]
- 21.Young JW, Burgess AR, Brumback RJ, et al. Pelvic fractures: value of plain radiography in early assessment and management. Radiology 1986; 160: 445–451. [DOI] [PubMed] [Google Scholar]
- 22.Meighan A, Gregori A, Kelly M, et al. Pelvic fractures: the golden hour. Injury 1998; 29: 211–213. [DOI] [PubMed] [Google Scholar]
- 23.Kregor PJ, Routt MLC, Jr. Unstable pelvic ring disruptions in unstable patients. Injury 1999; 30 (Supp 2):SB19–28. [DOI] [PubMed] [Google Scholar]
- 24.Ghanayem AJ, Wilber JH, Lieberman JM, et al. The effect of laparotomy and external fixator stabilization on pelvic volume in an unstable pelvic injury. J Trauma 1995; 38: 396–401. [DOI] [PubMed] [Google Scholar]
- 25.Palmer S, Fairbank AC, Bircher M. Surgical complications and implications of external fixation of pelvic fractures. Injury 1997; 28: 649–653. [DOI] [PubMed] [Google Scholar]
- 26.Branney SW, Moore EE, Cantrill SV, et al. Ultrasound-based key clinincal pathway reduces the use of hospital resources for the evaluation of blunt abdominal trauma. J Trauma 1997; 42: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 27.Rozycki GS, Ballard RB, Feliciano DV, et al. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg 1998; 228: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulanger BR, McLellan BA, Brenneman FD, et al. Prospective evidence of the superiority of a sonography-based algorithm in the assessment of blunt abdominal injury. J Trauma 1999; 47: 632–637. [DOI] [PubMed] [Google Scholar]
- 29.Burgess AR, Eastridge BJ, Young JWR, et al. Pelvic ring disruptions: effective classification system and treatment protocols. J Trauma 1990; 30: 848–856. [PubMed] [Google Scholar]
- 30.Cryer HM, Miller FB, Evers BM, et al. Pelvic fracture classification: correlation with hemorrhage. J Trauma 1988; 28: 973–980. [PubMed] [Google Scholar]
- 31.Hamill J, Holden A, Paice R, et al. Pelvic fracture pattern predicts pelvic arterial haemorrhage. Aust NZ J Surg 1999; 70: 338–343. [DOI] [PubMed] [Google Scholar]
- 32.Biffl WL, Moore EE, Offner PJ, et al. “Secondary” abdominal compartment syndrome in the general surgery patient: A highly lethal event. Presented at the 53rd Annual Meeting of the Southwestern Surgical Congress, April 2001.
- 33.Maxwell RA, Fabian TC, Croce MR, et al. Secondary abdominal compartment syndrome: an underappreciated manifestation of severe hemorrhagic shock. J Trauma 1999; 47: 995–999. [DOI] [PubMed] [Google Scholar]
- 34.Marvin RG, McKinley BA, Cocanour CS, et al. Factors associated with abdominal compartment syndrome during large-volume shock resuscitation. J Trauma 2000; 48: 199. [Google Scholar]