Abstract
Objective
To assess the demographics and characteristics of infections in surgical patients to define areas that deserve emphasis in surgical education.
Summary Background Data
As a result of evolving technology and diseases, the complexity of diagnosing and treating infections has increased during the past three decades for all patients, including those treated primarily by surgeons. No comprehensive analysis of these conditions in a single surgical cohort has been recently published.
Methods
The authors conducted a prospective, observational study of all infections occurring on the general and trauma surgery services at a single university hospital during a 3.5-year period.
Results
The authors identified 2,457 infections: 608 community-acquired, 1,053 occurring on the wards, and 796 occurring in the intensive care unit. Although dependent on patient location, the most common sites were abdomen, lung, and wound; the most common isolates were Staphylococcus epidermidis, Staphylococcus aureus, and Candida albicans; and the most commonly used antibiotics were ciprofloxacin, vancomycin, and metronidazole. The overall death rate was 13%, ranging from 5% after community-acquired infections to 25% after infections acquired in the intensive care unit.
Conclusions
Most infections treated by surgeons are hospital-acquired. Infections with gram-positive cocci and fungi are common, with pulmonary infections becoming more common. Fluoroquinolones have become important therapeutic agents. Depending on the type of practice, these data should be helpful to direct educational efforts so that surgeons can remain knowledgeable and active in the nonsurgical care of their patients.
Infections treated by general surgeons have traditionally been viewed as those needing surgical intervention, such as a boil or a perforated hollow viscus, or those complicating surgery, such as a wound infection. As the patient population throughout medicine has become older, more ill, and more complex, the range of infections treated by surgeons and occurring in surgical patients has likewise broadened significantly.
The kinds of infections, causative organisms, and therapeutic options have changed over time as well. Pathogens resistant to most known antibiotics are now common, but the number of individual new antibiotics, not to mention whole families of antibiotics, has also continued to increase. The frequency with which these pathogens infect surgical patients and these newer antibiotics are used by surgeons is poorly defined. Although the medical treatment of infections (emphasizing antibiotic use) is clearly ancillary to proper surgical technique, it is prudent for surgeons to maintain a working knowledge of these issues to provide optimal care under a wide variety of circumstances.
The object of this observational study, therefore, was to survey the relative frequency of different infections, pathogens, and use of antibiotics among a mixed general surgery population as we enter the 21st century. We hope that a clear outline of these data will be useful to the practicing surgeons as well as potentially acting as a guide for the education of those still undergoing surgical training.
METHODS
This study was approved by the University of Virginia Human Investigation Committee and conducted at the University of Virginia Health Sciences Center from December 15, 1996, to May 17, 2000. Because of the observational nature of the study, the need for informed consent was waived. The University of Virginia Health Sciences Center is a 750-bed rural teaching hospital that admits a fairly even distribution of primary, secondary, and tertiary care patients, includes a level 1 trauma center, and is the major referral center for the western half of Virginia and some areas of West Virginia. There are two surgery/trauma wards (total of 65 beds) and a 10-bed surgical/trauma intensive care unit (ICU).
All patients on the adult general and trauma surgery units were evaluated. Patients with infection were identified by every-other-day chart review, house staff/attending interview, and review of daily antibiotic usage and laboratory and microbiologic data by the investigators. More than 95% of the data were collected by a single investigator (R.G.S.) during the entire period of study to ensure consistent data collection.
Centers for Disease Control and Prevention definitions 1 were used, with the exception of catheter-related infections (see below). Criteria for the diagnosis of pneumonia included systemic evidence of infection, purulent sputum production, isolation of a predominant organism from an appropriately obtained culture, and a new or changing infiltrate or effusion on chest radiograph. Diagnosis of a urinary tract infection (UTI) required the isolation of greater than 105 organisms per milliliter of urine or greater than 104 organisms per milliliter with symptoms. Bloodstream infections were diagnosed by isolation of organisms from any sterilely obtained blood culture, with the exception of Staphylococcus epidermidis or other coagulase-negative staphylococci, which required isolation from two separate sites. Catheter-related infections were identified by isolation of 15 or more colony-forming units from catheter tips by semiquantitative roll plate technique in the setting of clinical infection. We have found that under these circumstances, bloodstream infection is not an independent predictor of outcome after multivariate analysis. 2 Thus, positive blood cultures were not required for the diagnosis of catheter-related infections, and patients were treated similarly regardless of diagnosis of bloodstream infection. Antibiotic-impregnated catheters were not used during the entire length of the study. Cellulitis, peritoneal infections, and surgical site infections were generally diagnosed clinically, frequently without cultures.
Infections were considered nosocomial if they were not documented or suspected at the time of admission. 1 Infectious episodes occurring more than 72 hours apart in the same patient were considered separately and individually for analysis. Antibiotic-resistant aerobic gram-positive cocci were defined as methicillin-resistant Staphylococcus aureus or S. epidermidis and gentamicin- or vancomycin-resistant enterococcal species. Antibiotic-resistant aerobic gram-negative rods were defined as any gram-negative bacillus resistant to one or more of the following: all aminoglycosides, including amikacin; all third- and fourth-generation cephalosporins; all fluoroquinolones; all carbapenems. A finding of “mixed flora” was not counted either as gram-positive or gram-negative. Organisms identified but not speciated (e.g., “GNR, no further speciation”) were considered sensitive.
Intake variables recorded at the time of diagnosis for each infectious episode included age, gender, race, white blood cell count (WBC), temperature, Acute Physiology and Chronic Health Evaluation score (APACHE II), 3 date of admission, and the time from admission and diagnosis of infection until treatment initiation. The WBC, temperature, and APACHE II score were all the most extreme values recorded within the first 24 hours of diagnosis of infection. Other variables recorded included infection site, culture data, antibiotic regimen, duration of antibiotic treatment, and presence of significant preexisting comorbidities, including diabetes mellitus (type I or II), chronic renal insufficiency (serum creatinine ≥2.0 mg/dL [176.8 μmol/L] before admission), mechanical ventilator dependency (excluding the immediate postoperative period, ≤48 hours), hemodialysis dependency, coexisting malignancy, corticosteroid therapy, blood transfusion (administration of nonautologous cellular blood products after admission but before diagnosis of infection), pulmonary disease, cardiovascular disease, and liver disease.
Outcomes studied included duration of antibiotic use, total hospital length of stay, hospital length of stay after initiation of treatment for infection, time from initiation of treatment for infection until defervescence (maximum temperature <38°C for 24 consecutive hours), return to normal of WBC (≤11,000/mm3), and death before discharge. Death before discharge while still receiving antibiotic therapy was also noted as a crude estimate of the deaths attributable to infection.
Univariate analysis of categorical data was performed using chi-square testing, and continuous variables were analyzed using two-tailed Student t tests with equal or unequal variances based on analysis by the F test. Values are expressed as mean ± standard error (continuous variables) or percentage of the group of origin (categorical variables). All probability values are two-tailed and, per Bonferroni, P ≤ 0.015 was considered significant when each one of the three groups (community-acquired infections, ward infections, and ICU infections) was compared with all infections. Statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC) and GB-STAT Version 6.5 (Dynamic Microsystems, Silver Spring, MD).
RESULTS
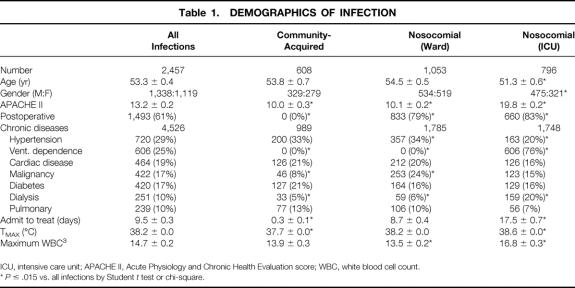
During the 3.5 years of the study, 4,385 general surgery and 1,750 trauma admissions were followed. A total of 2,457 separate infections were treated in 1,218 patients, or 2.0 infections per infected patient. Demographics for all patients and categorized by patient location at the onset of infection (home/community-acquired, hospital ward, or ICU) are given in Table 1. Patients with an infection clearly associated with surgery (e.g., wound infection) who were discharged home but readmitted for treatment of that infection were included in the nosocomial-ward group. A total of 1,849 infections were nosocomial for a crude attack rate of 30.1 infections per 100 admissions. ICU patients were younger, were more frequently male (60% vs. 54%), were more likely to be dialysis-dependent, and had a higher severity of illness (APACHE II score) than the total cohort. Interestingly, these patients also had a higher maximum temperature and WBC at the time of treatment, showing their ability of mounting a physiologic response to infection.
Table 1. DEMOGRAPHICS OF INFECTION
ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation score; WBC, white blood cell count.
*P ≤ .015 vs. all infections by Student t test or chi-square.
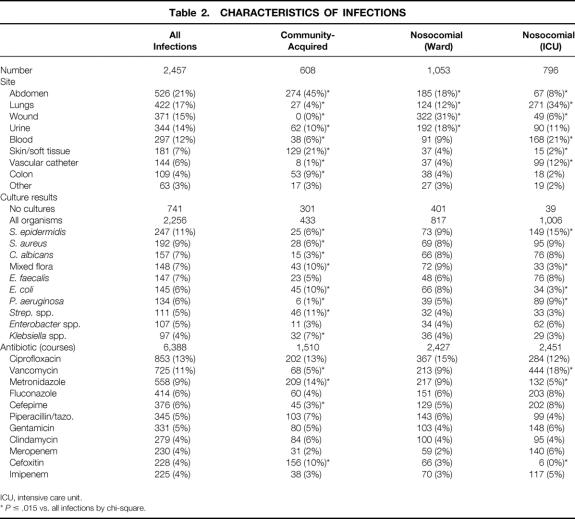
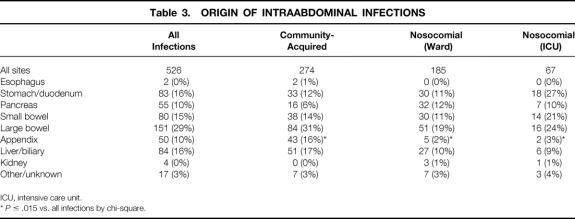
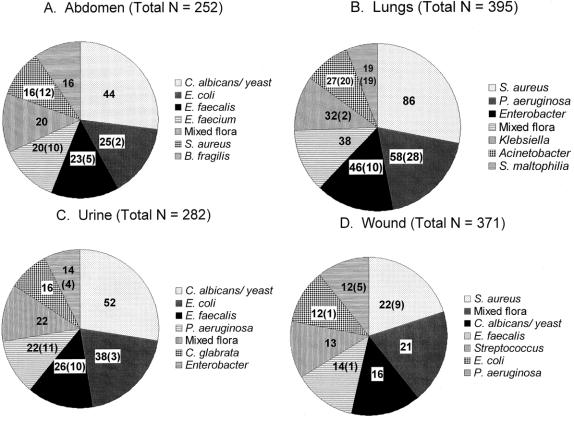
Table 2 gives characteristics of identified infectious episodes. Overall, intraabdominal infections were most frequent, although this was highly dependent on patient location: wound infections were most common in ward patients and pulmonary infections were most common in the ICU. The breakdown of abdominal infections by organ of origin is given in Table 3. The colon continues to be the predominant source of these infections. Surprisingly, the stomach/duodenum was the most common site of origin for nosocomial abdominal infections, the majority of which could be attributed to anastomotic leaks or dislodged gastrostomy tubes. Nearly half of all community-acquired and ward infections were treated without cultures. Sixty-five organisms in all were isolated from surgical patients. S. epidermidis (coagulase-negative Staphylococcus) was the single most common isolate (11%), although seven other organisms were found in 5% or more of infections, and the organisms treated were highly dependent on patient location. Among the most common individual sites, Escherichia coli was the most common isolate from the abdomen, S. aureus was the most common lung isolate, and S. epidermidis was the most common bloodstream isolate. Because most of the infections were nosocomial and would thus be expected to exhibit changes in ecology compared with historical data, the seven most commonly isolated hospital-acquired pathogens by site (and the number considered antibiotic-resistant) are shown in Figure 1. In addition, 75 of the 252 (28%) abdominal infections, 27 of the 395 (7%) pulmonary infections, 46 of the 282 (16%) urinary tract infections, and 254 of the 371 (68%) wound infections were treated without culture.
Table 2. CHARACTERISTICS OF INFECTIONS
ICU, intensive care unit.
*P ≤ .015 vs. all infections by chi-square.
Table 3. ORIGIN OF INTRAABDOMINAL INFECTIONS
ICU, intensive care unit.
*P ≤ .015 vs. all infections by chi-square.
Figure 1. Most common pathogens isolated from nosocomial infections by site. Figures in parentheses represent the number of isolates that were considered antibiotic-resistant by in vitro testing. (A) Abdominal infections. (B) Pulmonary infections. (C) Urinary tract infections. (D) Wound infections.
A total of 6,388 courses with 57 different antibiotics were used during the study period, or a mean of 2.6 antibiotics used to treat each infection. A wide and fairly even distribution across the major families of antibiotics was noted: penicillins, 1,016 courses; fluoroquinolones, 1,000 courses; cephalosporins, 854 courses; vancomycin class, 725 courses; all antifungals, 668 courses; metronidazole class, 558 courses; carbapenems (imipenem and meropenem), 455 courses; and aminoglycosides, 487 courses. Ciprofloxacin was the most commonly used single antibiotic, followed by vancomycin and metronidazole. Other than a large amount of vancomycin use among ICU patients and cefoxitin use among patients with community-acquired infections, there was a surprising similarity of frequency of use of antibiotics across all patient groups.
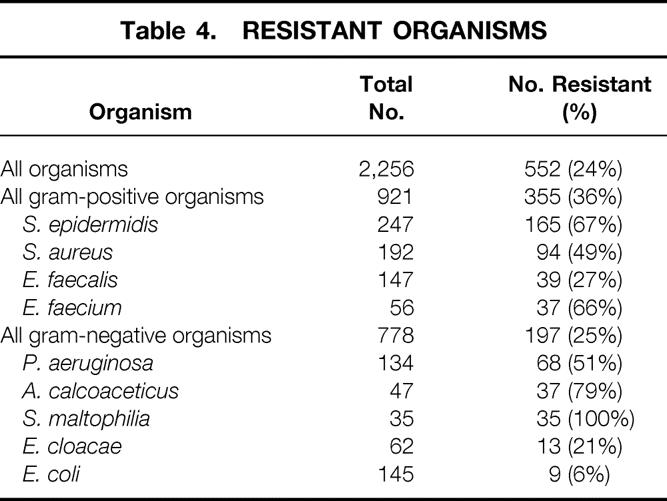
A high percentage of isolates were antibiotic-resistant: 355 of the 921 (36%) gram-positive organisms and 197 of the 778 (25%) gram-negative organisms (Table 4). As expected, most of these episodes occurred in ICU-bound patients (65%), with 24% and 11% occurring on the wards and in community-acquired infections, respectively. Only eight isolates (Stenotrophomonas maltophilia, n = 5;Pseudomonas aeruginosa, n = 1;Burkholderia cepacia, n = 1;Enterococcus faecium, n = 1) were confirmed to be resistant to all commercially available antibiotics by in vitro testing.
Table 4. RESISTANT ORGANISMS
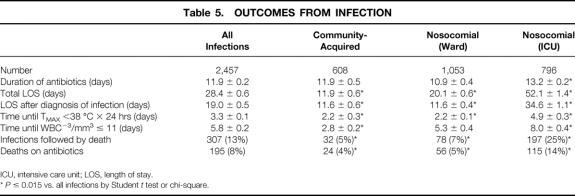
Outcomes from infection are outlined in Table 5. Overall, although 307 of 2,457 episodes of infection were followed by death (13%), 104 of 1,218 patients treated for infection died (9%). As expected, infectious episodes occurring in the ICU were associated with a longer antibiotic course, overall hospital length of stay, length of stay from initiation of treatment for infection until discharge, hospital death rate, and attributable death (death while still receiving antibiotics). Patients in the ICU also took significantly longer to resolve their fever and leukocytosis if present at the time of diagnosis.
Table 5. OUTCOMES FROM INFECTION
ICU, intensive care unit; LOS, length of stay.
*P ≤ 0.015 vs. all infections by Student t test or chi-square.
DISCUSSION
Although the management of infection continues to be a relatively minor part of the education of surgeons, the associated death rate (9%) is significantly higher than for any elective procedure, and most emergent ones as well. In addition, the changes in the nature of infectious diseases and the drugs used to treat them are reflected in surgical patients, and this evolution cannot be ignored by surgeons if they intend to treat their patients effectively, autonomously, and optimally. The object of the current study was to define and emphasize important points in the medical management of surgical infections.
Our results show that general surgeons continue to take care of a wide variety of infectious disease problems. Whether the overall results presented here are similar to those that could be generated at other hospitals is unclear, although it is likely that the trends seen based on the patient location at the time of onset of infection (home, hospital ward, or ICU) are broadly applicable. Interestingly, it now appears that most infections treated by surgeons are nosocomial and occur in patients with a moderately high severity of illness, even if those presenting in the ICU are excluded. Because of this, it would appear necessary to continue to maintain a working knowledge of risk factors for and treatment of hospital-acquired infections. Despite the complexity of these conditions, however, we have previously reported that patients on our services are treated with appropriate initial therapy approximately 75% of the time, and 99% received appropriate therapy after a mean of 1 day of treatment. 4 Because medical infectious disease consultation was rare, the implication is that these processes can be successfully managed by surgical residents and staff with the input of surgeons with an interest in this field.
Demographically, although it was not unexpected that our ICU population was younger than other infected patients (ostensibly a result of trauma admissions), the relative overrepresentation of male patients in the ICU was surprising but not unprecedented. Bone 5 noted in 1992 a significantly higher number of male versus female patients enrolled in multiple sepsis studies, and Wichmann et al 6 found a higher incidence of severe sepsis or septic shock among 4,218 ICU patients in Germany. A higher incidence of infection among male trauma patients in the ICU was also reported by Offner et al 7 and Oberholzer et al. 8 Although infections may develop in women less frequently, some data suggest women also have a higher death rate from nosocomial infections, particularly pneumonia. 9 It is further notable that this same infected ICU population had a higher temperature and WBC than other groups, indicating these patients are quite capable of mounting a robust systemic response. This finding, however, also raises the question of whether these infections were first treated relatively late in their natural course, leading in part to the significantly worse outcomes found for them.
A wide variety of infecting organisms was found, with distribution highly dependent on patient location and site of infection. C. albicans and yeast, for example, were the most common pathogens isolated from nosocomial abdominal and urinary infections but were rare among all community-acquired infections. Overall, the 10 most common specific pathogens represented only 70% of all organisms cultured. However, nearly half of all community and ward infections were treated without cultures, although given the predominant diseases (community-acquired abdominal and postoperative wound infections), this is almost certainly appropriate. Episodes with resistant bacteria were relatively common (24% of all organisms, 20% of all episodes), with a predominance in the ICU population. Nonetheless, 35% of these infections occurred outside the ICU, indicating that even surgeons who do not directly treat critically ill patients need some knowledge of this issue. Finally, it is encouraging that only a total of eight isolates were resistant to all available antibiotics (0.3%), suggesting the “postantibiotic era” (when completely drug-resistant pathogens are predicted to run rampant) is not imminent. However, organisms that are resistant to multiple, but not all, antibiotics have been associated with an increased death rate, as we recently reported for gram-positive organisms, 10 and continued efforts to try to control their spread are probably warranted.
Many different antibiotics were used during the study period, and the use of multiple simultaneous or sequential agents was common (2.6 antibiotics/infection). Because this study examined therapy rather than prophylaxis, it almost certainly remains true that cephalosporins (including cefazolin and cefoxitin used before surgery for elective cases) are the antibiotics used most frequently by surgeons. The evolution of infectious diseases and the pharmaceutical industry, however, has apparently shifted therapeutic drug use away from penicillins and cephalosporins toward newer agents such as fluoroquinolones, despite little evidence regarding significant benefits in terms of efficacy or cost. Rather, other factors, such as a better side effect profile, easier dosing regimens, and better compliance or improved oral bioavailability may be important in determining utilization patterns. It is also clear that physicians who care for critically ill inpatients need to keep current on the ever-increasing number of antifungal agents currently on the market or soon to be introduced. Surgeons should be aware of these issues to treat their patients in an optimally efficient, cost-effective, and patient-friendly manner.
How can surgeons reduce the death and complication rates of infections in their patients? Fortunately, the “postantibiotic era,” where pathogens are resistant to all known antibiotics, does not appear to be close at hand, because less than 1% of organisms isolated fit this category. Nevertheless, even with effective antibiotics, the death rate from these diseases is still too high, and it is probably through prevention that the greatest improvement in overall surgical patient outcome can be made. For example, extensive guidelines have recently been formulated to help prevent wound infections, 11 and many of the principles underlying these recommendations have been thoroughly investigated. 12 Because most of the infections we identified were nosocomial, and only a minority of those were localized to the surgical site, increased efforts to prevent infections at other sites in inpatients must be undertaken as well. Examples of this preventive strategy include proper positioning to reduce the chance of pneumonia, careful central venous catheter care, and avoidance of invasive devices, such as bladder catheters, whenever possible. More general infection control measures, such as frequent handwashing and isolating patients with resistant bacterial infections, will also be necessary to reduce infection rates as much as possible.
Finally, although these data suggest guidelines for teaching regarding the medical management of surgical infections, this topic remains an ancillary body of knowledge. The emphasis for the education of surgeons (and probably more importantly nonsurgeons) must remain on the primary role of surgical management, including adequate drainage, source control, and the effective use of nonoperative/percutaneous techniques. Regardless of any advances in antimicrobial therapy, the abandonment of these key principles can only result in treatment failure in what is an increasingly elderly, ill, and challenging patient population.
Discussion
DR. DAVID N. HERNDON (Galveston, Texas): Thank you, Dr. Pruett, on making a detailed review quite clear in your presentation. This detailed review of infections and antibiotic utilization and treatment of those infections by a single surgical department over 3–5 years provides an insightful resource that indicates critical changes in surgical practice. The most commonly used antibiotics for treatment by surgeons in Charlottesville are now ciprofloxacin, vancomycin, metronidazole and fluconazole, a major departure from the previously used cephalosporins, aminoglycosides, Clindamycin, and amphotericin.
The incidence of strains of bacteria resistant to antibiotics is startling: 67% of Staph epidermidis, 50% of Staph aureus, 60% of enterococci, 50% to 100% of Pseudomonas, Acinetobacter, and the hard-to-pronounce Stenotrophomonas maltophilia. Candida accounts for an increasing number of surgical infections. Thirteen percent of surgical infections contributed to mortality, with that number being 25% in ICU patients, a disturbingly high 30/100 patients developed nosocomial infections.
The authors leave no question that the pattern of surgical infections and their treatment are rapidly and perilously changing and that surgeons must critically focus on these changes.
The questions raised by this paper are:
What is best practice? And how can the information available in this unique data set be used to improve practice? Two to six antibiotics were used to treat each infection. In what percent of cases were these the correct antibiotics to use? In what percent of cases were the most effective least expensive and least likely drugs to develop resistance used? Were cultures taken as often and as soon as they should have been? Were antibiotics adjusted in a timely fashion in response to sensitivities? Should the use of antibiotics by surgeons be controlled by experts in surgical infections to slow these disturbing trends revealed in this review?
Why do you think 27% of intraabdominal abscesses in this study came from the duodenum and stomach? Are we moving beyond the antibiotic era? Where should we go?
How representative do you think special university is of the nation?
Thank you.
DR. BASIL A. PRUITT, JR. (San Antonio, Texas): I rise to compliment Dr. Pruett on a very important study and to ask three questions.
You mentioned using your data to pick empiric therapy. In the case of a pulmonary infection Staph aureus is the most common single offender, but the gram-negatives overall are the most common causative agents. So do I pick gram-positive or gram-negative antibiotics for this patient that I have made the diagnosis in?
Secondly, you seem to imply that antibiotic resistance is somehow of clinical importance. We have not found that to be true. Multiply resistant staph are just staphylococcus by another name. Do you have any documentation that it really has a significant comorbid effect above what that patient would have based on age and disease?
Thirdly, when you get an endemic resistant strain, how do you deal with it? We have found cohort nursing is very effective in eliminating such from our ICUs. Is that your experience as well?
I thank the Association for the privilege of the floor.
DR. ROBERT SAWYER (Charlottesville, Virginia): Thank you very much. I appreciate all the excellent questions and the opportunity to give you our data.
In regards to Dr. Herndon’s questions: The question about what is best practice, that is, as for any other medical question, difficult and ever-changing. The purpose for this was to at least have some broad guidelines in what we think is important throughout the country, but to also analyze our own very specific data in our own hospital. And we take this data back and use it to modify many of our empiric antibiotic treatment regimens.
We have actually looked up the percentage of correct antibiotics. Our empiric choices, in our hospital at least, are correct about 70% of the time. And after a 2- to 3-day period, at least by in vitro testing, it is correct about 98% of the time.
We culture most of our nosocomial infections. The largest group of noncultured infections are intraabdominal infections which come in from the community. We have had a hard time figuring out whether those are cost-effective or not. Our gut feeling is, pardon the pun, is that they are not cost-effective, so we do not routinely do those.
Whether experts should dictate our antibiotic use, that is an enormous question for anyone who is interested in surgical infections. The antibiotics should not be dictated by experts, particularly if the experts are medical infectious disease experts. We have had a series and will continue to have a series of run-ins with our medical colleagues, at least at our hospital, because they really don’t understand the overall nature of our patient characteristics. It is useful, we think, to identify somebody in any hospital who is a surgeon who has at least some interest in this area to be able to make some recommendations frequently.
In terms of duodenal and stomach as origin for nosocomial infections, the majority of those are patients who had repairs for perforations that subsequently developed a small leak and then were treated with antibiotics.
Are we in the postantibiotic era? That concept that we will get into a time point in medical care where we have rampant infections with organisms which are resistant to all known antibiotics certainly has made the cover of major news magazines recently. We don’t really think that is going to be the case. Of all the organisms that we have ever treated, it comes down to somewhere less than 0.1 of 1% fall into that category. And, as you can imagine, those are in critically ill patients, and their mortality is probably related to the fact that they have been on multiple long courses of antibiotics for multiple problems.
How representative our data is, is an excellent question, particularly regarding generalizability of the data. And the answer is, I don’t really know. I think one of the reasons we broke down our infections based on community-acquired or ward-acquired, or occurring on the ward infections, and ICU infections, is so that surgeons can look at the data and look at their own practice and try to figure out where their practice most commonly lies.
Then, finally, for Dr. Pruitt’s questions in terms of empiric therapy for pneumonia, we generally start with a gram-negative agent. If there is any evidence of gram-positive organisms in the sputum, then we will add vancomycin because of a high incidence of methicillin-resistant staph.
Is antibiotic resistance important? We struggle with that. Is using one antibiotic versus another, does that make a difference? We actually have just submitted an abstract to the Surgical Infection Society, where we purport to show, Dr. Pruitt – and hopefully you will be able to comment– that resistant gram-negative infections, even controlling for severity of illness, nosocomial origin, and so forth, may have a higher mortality.
And then, finally, what do we do with our endemic strains? We have tried cohort nursing in our hospital where we have a very high utilization of beds. That has not been possible, and we have been left to rely on the old gowns and gloves and things which our epidemiologists like, but I am really completely uncertain are really very effective.
Thank you very much.
Footnotes
Presented at the 112th Annual Meeting of the Southern Surgical Association, December 4–6, 2000, Palm Beach, Florida.
Supported by a Surgical Infection Society Junior Faculty Grant.
Correspondence: Dr. Robert G. Sawyer, Box 800709, University of Virginia HSC, Department of Surgery, Charlottesville, VA 22908-0709.
E-mail: rws2k@virginia.edu
Accepted for publication December 2000.
References
- 1.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128–140. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier SJ, Crabtree TD, Gleason TG, et al. Bacteremia associated with central venous catheter infection is not an independent predictor of outcomes. J Am Coll Surg 2000; 190: 671–680. [DOI] [PubMed] [Google Scholar]
- 3.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 4.Pelletier SJ, Crabtree TD, Gleason TG, et al. Waiting for microbiologic data to direct therapy against nosocomial infections in febrile surgical patients: are outcomes worsened? Arch Surg 1999; 134: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 1992; 268: 3452–3455. [PubMed] [Google Scholar]
- 6.Wichmann MW, Inthorn D, Andress HJ, et al. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med 2000; 26: 167–172. [DOI] [PubMed] [Google Scholar]
- 7.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg 1999; 134: 935–938. [DOI] [PubMed] [Google Scholar]
- 8.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma 2000; 48: 932–937. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree TD, Pelletier SJ, Gleason TG, et al. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA 1999; 282: 2143–2148. [DOI] [PubMed] [Google Scholar]
- 10.Gleason TG, Crabtree TD, Pelletier SJ, et al. Prediction of poorer prognosis by infection with antibiotic-resistant gram-positive cocci than by infection with antibiotic-sensitive strains. Arch Surg 1999; 134: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 11.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999; 27: 97–132. [PubMed] [Google Scholar]
- 12.Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med 1992; 326: 281–286. [DOI] [PubMed] [Google Scholar]