Abstract
Aquaporin-2 (AQP2) is the predominant vasopressin-regulated water channel in kidney connecting tubule (CNT) and collecting duct (CD) and is essential for renal regulation of body water balance. However, the relative role of AQP2 to urinary concentration in the CNT and CD segments is unknown. To examine this directly, transgenic mice expressing AQP2 selectively in CNT but lacking AQP2 expression in CD (AQP2-CD-KO) and mice lacking AQP2 globally (AQP2-total-KO) were generated by exploiting the Cre/loxP technology. LoxP sites were inserted into AQP2 introns 2 and 3, and transgenic mice were bred with strains expressing Cre recombinase under the control of CD-specific Hoxb7− or global EIIa promoter. Mice lacking AQP2 globally died postnatally (days 5–12). AQP2-CD-KO mice were viable to adulthood and showed decreased body weight, 10-fold increased urine production (0.96 ± 0.11 vs. 0.10 ± 0.01 ml/g of body weight), and decreased urinary osmolality (170 ± 19 vs. 1,630 ± 135 milliosmoles/kg of H2O). Immunohistochemical staining of AQP2-CD-KO kidneys (n = 12) revealed sustained, strong AQP2 expression in CNT cells, whereas >95% of CD principal cells were completely AQP2-negative. Water deprivation for 3 hours caused only marginal decreased urine output (87 ± 7% of levels when mice had free water access; P = 0.04) with no change in urine osmolality, revealing an absence of compensatory mechanisms. These results demonstrate that AQP2 in CNT is sufficient for postnatal survival and that AQP2 in CD is essential for regulation of body water balance and cannot be compensated for by other mechanisms.
Keywords: connecting tubule, kidney, vasopressin, water channel
Aquaporins (AQPs) in the kidney are essential for body water balance regulation. AQP, expressed in the proximal nephron, is essential for reabsorption of the large fraction of water filtered in the glomerulus. Three AQPs are expressed in the collecting duct (CD) and connecting tubule (CNT): AQP2, AQP3, and AQP4. They are involved in the vasopressin-regulated water reabsorption for tight control of body water balance. AQP2 is expressed in the apical plasma membrane and vesicles in the connecting tubule cells and in the CD principal cells (1) and is the chief target for regulation of the osmotic water permeability of these segments in response to vasopressin. This water balance regulation occurs by short-term, acute regulated translocation of intracellular AQP2-bearing vesicles to the apical plasma membrane (2–5) and by long-term regulation of the expression (reviewed in ref. 6). This process allows production of concentrated urine and is essential for water balance regulation. The molecular mechanisms involved in AQP2 insertion into the plasma membrane are the subject of intensive research and are not completely understood (recently reviewed in ref. 6).
Studies in the rat indicate similar regulation of AQP2 gene expression and intracellular trafficking in the CNT and in the CD under various physiological and pathophysiological conditions (7), indicating that similar water transport regulation mechanisms exist in these segments. However, the relative role of AQP2 in the CNT segment and CD subsegments in the urinary concentrating process has not been directly determined. This role can now be determined in transgenic mice with cell- or organ-specific gene knockout generated by employing the Cre/loxP system.
In the present report we describe the generation of global AQP2-knockout mice (AQP2-total-KO) and tissue-specific AQP2-knockout mice with sustained AQP2 expression in the CNT but lacking AQP2 expression in most of the CD cells (AQP2-CD-KO). To generate these mice, two loxP sites were inserted into the AQP2 gene introns 2 and 3. Activity of the Cre recombinase (expression regulated by global promoter EIIa or CD-specific Hoxb7 promoter) leads to deletion of the AQP2 gene exon 3 containing one of the conserved NPA (Asn-Pro-Ala) motifs that form the channel pore and results in the destruction of channel function.
Results
Generation of AQP2-Total-KO Mice.
The “total” deletor EIIa-Cre strain showed low and variable Cre recombination efficiency, resulting in only a small fraction of “floxed” AQP2 allele (termed AQP2flx) being converted to an AQP2 allele with a deleted exon 3 (termed AQP2ΔE3) in the DNA from tail biopsy (confirmed by PCR genotyping). Therefore, mice with germ-line transmission of the AQP2ΔE3 allele were identified (AQP2flx/ΔE3EIIa-Cre−) and subsequently bred to obtain AQP2-total-KO mice (AQP2ΔE3/ΔE3) (Fig. 1). The AQP2-total-KO mice were similar in weight to their healthy siblings at birth but failed to thrive and died within 2 weeks of age (Fig. 2). The kidneys from AQP2-total-KO pups showed papillary atrophy and an increase in pelvic space, i.e., signs of hydronephrosis development. Immunoblotting showed the disappearance of nonglycosylated and glycosylated AQP2 bands and showed the appearance of a novel, 23-kDa band in the AQP2ΔE3/ΔE3 mice, representing the nonglycosylated, truncated AQP2 (results not shown).
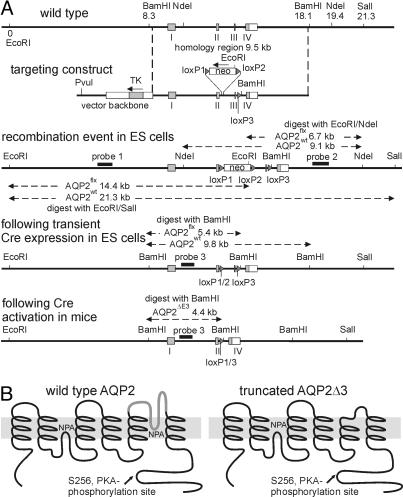
Fig. 1.
Targeted disruption of the mouse AQP2 gene. (A) Strategy for generating AQP2flx/flx mice. (B) Predicted peptide structures of wild-type AQP2 before (Left) and after (Right) deletion of exon 3. Deletion eliminates most of transmembrane domain 5, including the conserved NPA (Asn-Pro-Ala) motif in the channel-forming part of AQP2.
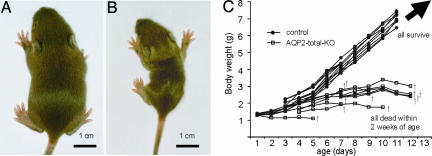
Fig. 2.
Growth retardation of AQP2-total-KO mice. A 10-day-old wild-type mouse (A) and a AQP2-total-KO mouse (B) from the same litter. The AQP2-total-KO mouse grew markedly less. (C) The growth of AQP2-total-KO and wild-type mice over time. All AQP2-total-KO mice died before day 13, whereas all controls survived.
Immunohistochemical labeling of AQP2 in kidney sections from 10-day-old AQP2-total-KO mice revealed weak cytoplasmic labeling in the CNT and CDs compared with strong AQP2 labeling in sections of kidneys from control siblings (Fig. 3), indicating reduced stability and increased degradation of the truncated AQP2 protein (assuming equally efficient mRNA expression). The labeling was exclusively associated with the cytoplasm, in contrast to the strong apical staining of AQP2 in control siblings and consistent with retention of misfolded mutant AQP2 protein in the endoplasmic reticulum. Immunohistochemical labeling using p-AQP2 (AQP2 phosphorylated at Ser-256) antibodies revealed weak cytoplasmic labeling in the CNTs and CDs in AQP2-total-KO mice expressing the truncated AQP2, whereas strong apical AQP2 labeling was present in sections of kidneys from control siblings (results not shown). This labeling pattern indicates that despite the improper folding structure of the truncated AQP2 protein, it can undergo phosphorylation and that the AQP2 C terminus (containing Ser-256) is situated in the cytoplasm.
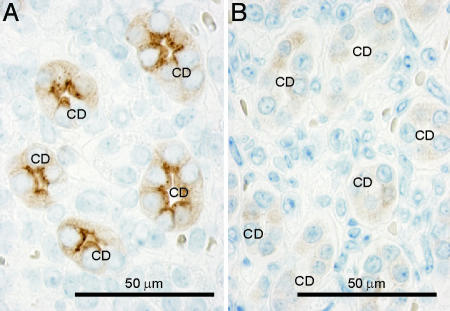
Fig. 3.
Immunohistochemical labeling of AQP2 in control (A) and AQP2-total-KO (B) mice. In kidneys from control mice, strong apical labeling is associated with CD principal cells (CD). In contrast, weak and diffuse cytoplasmic labeling was observed in kidney CD principal cells in AQP2-total-KO mice, indicating the presence of low levels of truncated AQP2ΔE3 protein.
Generation of AQP2-CD-KO Mice.
AQP2flx/flx mice were bred with Hoxb7-Cre mice, which express Cre recombinase exclusively in the CD epithelium of the kidney and in the epithelium in parts of the urinary tract (8). The AQP2flx/flxHoxb7-Cre+ genotype was confirmed by PCR. The AQP2flx/flxHoxb7-Cre+ mice (termed AQP2-CD-KO) showed increased postnatal survival compared with AQP2-total-KO mice, although some of the AQP2-CD-KO pups died before weaning. The AQP2-CD-KO mice that survived to adulthood were significantly smaller than the control (AQP2flx/flxHoxb7-Cre−) siblings, and the kidneys showed progressive papillary atrophy and hydronephrosis. The degree of these changes varied among AQP2-CD-KO mice.
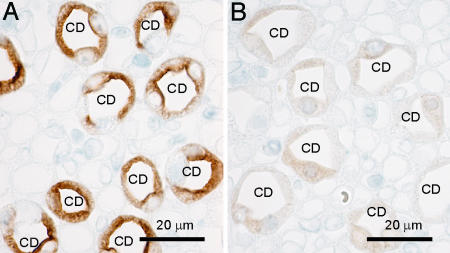
Immunohistochemistry revealed sustained AQP2 expression in the CNT and an almost complete lack of AQP2 expression in the CDs (Fig. 4). These findings were confirmed by double immunofluorescence labeling with AQP2 and a distal convoluted tubule and CNT marker calbindin D-28k (Fig. 5). In the majority of cells in the CDs in AQP2-CD-KO mice, there was very weak cytoplasmic labeling, reflecting the truncated AQP2 protein product of the AQP2ΔE3 allele (Fig. 4 D and F). However, a few cells in the kidney CDs showed strong apical labeling and presumably represent cells where Cre recombination of both AQP2flx alleles was unsuccessful (Fig. 4F).
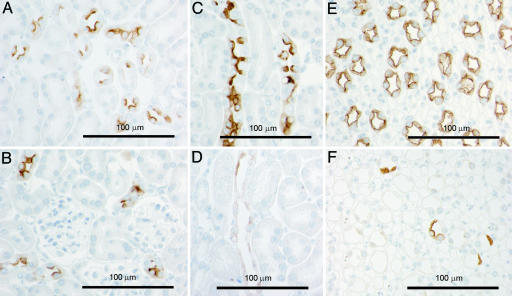
Fig. 4.
Immunohistochemical labeling of AQP2 in sections of kidneys from control (A, C, and E) and AQP2-CD-KO (B, D, and F) mice. A and B are from kidney cortex displaying CNTs. C and D are from kidney outer medulla displaying outer medullary CDs, and E and F are from kidney inner medulla displaying inner medullary CD. The CNT segment in the AQP2-CD-KO mice (B) expresses wild-type AQP2 protein.
Fig. 5.
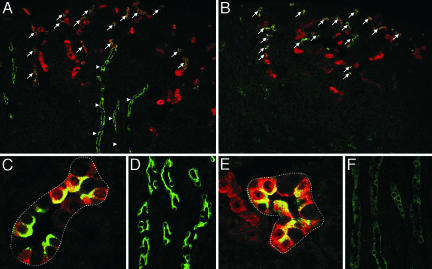
Immunofluorescence double labeling of AQP2 (green) and a distal convoluted tubule and CNT marker calbindin D-28k (red) in sections of kidney cortex from control (A, C, and D) and AQP2-CD-KO (B, E, and F) mice. Strong AQP2 labeling was present in the CNT of both animals (A and B, arrows), whereas only the control mouse displayed strong AQP2 labeling in the calbindin D-28k-negative cortical CDs (A, arrowheads). (C and E) Higher magnification of CNT. (D and F) Higher magnification of CDs.
Immunohistochemical labeling using antibody recognizing p-AQP2 revealed weak cytoplasmic labeling in the CDs in AQP2-CD-KO mice (Fig. 6B), comparable to the labeling observed in AQP2-total-KO mouse kidneys, indicating the presence of AQP2 phosphorylation of the mutant AQP2 protein. Strong apical AQP2 labeling was present in the CDs of kidneys from control siblings (Fig. 6A).
Fig. 6.
Immunohistochemical labeling of AQP2 phosphorylated at Ser-256 in sections of kidneys from control (A) and AQP2-CD-KO (B) mice. In kidneys from control mice, strong apical labeling was associated with CD principal cells (CD). In contrast, weak and diffuse cytoplasmic labeling was observed in CD in the AQP2-CD-KO mouse, indicating phosphorylation of the truncated AQP2ΔE3 protein.
Severe Urinary Concentrating Defect in AQP2-CD-KO Mice.
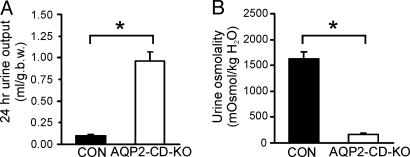
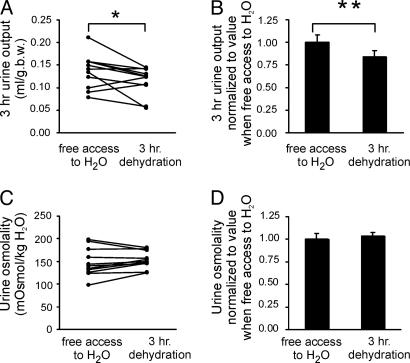
Twenty-four-hour urine samples were obtained by placing the mice in metabolic cages. As shown in Fig. 7, the AQP2-CD-KO mice exhibited a 10-fold increase in urine output compared with control mice (0.96 ± 0.11 ml/g of body weight for AQP2-CD-KO vs. 0.10 ± 0.01 ml/g of body weight for control, n = 5 per group). This observation was associated with a markedly reduced urine osmolality (170 ± 19 milliosmoles/kg of H2O for AQP2-CD-KO vs. 1,630 ± 135 milliosmoles/kg of H2O for control, n = 5 per group). As shown in Fig. 8A and B, 3 h of water deprivation of the AQP2-CD-KO mice resulted in an only marginal decrease in urine output (87 ± 7% compared with urine output when mice had free access to water; n = 11, P = 0.04), but urine osmolality remained unchanged and highly diluted (Fig. 8 C and D). This finding further demonstrated that the AQP2-CD-KO mice have a severe urinary concentrating defect and that this defect cannot be compensated for significantly.
Fig. 7.
AQP2-CD-KO mice had a markedly higher 24-h urine output compared with control mice (A) and markedly reduced urine osmolality compared with control mice (B). ∗, P < 0.05.
Fig. 8.
Changes in urine output (A and B) and urine osmolality (C and D) in response to free access to water or 3 h of complete water restriction of AQP2-CD-KO mice. The values were obtained in the same animals on consecutive days. Paired Student's t test reveals marginal, albeit statistically significant, reduction in urine output (∗, P = 0.04) but absence of significant differences in urine osmolality in response to 3 h of water restriction (B) (∗∗, P < 0.05).
Discussion
In the present study, we used the Cre/loxP strategy and generated transgenic mice lacking AQP2 globally (AQP2-total-KO) as well as mice expressing AQP2 selectively in the CNT but lacking AQP2 expression in the CD (AQP2-CD-KO mice). AQP2-total-KO mice failed to thrive and died postnatally because of an excessive loss of extracellular fluid volume. In contrast, the AQP2-CD-KO mice survived to adulthood, but they exhibited marked polyuria and severe urinary concentrating impairment in response to 3 h of water deprivation, indicating that AQP2 in the CD plays a key role in the regulation of body water balance. Because the CNT is not easily accessible by conventional physiological techniques, AQP2-CD-KO mice are ideal for use as an experimental animal model for directly studying the role of AQP2 selectively expressed in the CNT (but not in the CD) in the urinary concentrating process and regulation of body water balance. Moreover, this model demonstrates the critical role of AQP2 in the CD and CNT in the urinary concentration process and body water metabolism.
AQP2-Total-KO Mice.
We demonstrated that AQP2-total-KO mice failed to thrive and suffered from severe impairment of postnatal kidney development. This failure of growth was possibly due to massive contraction of extracellular fluid volume associated with polyuria and severe urinary concentrating defect. In contrast, AQP1-, AQP3-, or AQP4-deficient mice revealed a urinary concentrating defect but not neonatal mortality (9–11). This finding is consistent with the notion that apical AQP2 represents the rate-limiting factor for transcellular water transport in the CD and is also consistent with physiological studies in amphibian urinary bladder as well as in the mammalian CD that found that the apical plasma membrane water permeability is rate-limiting (see ref. 6 for review). This finding is also consistent with previous data from phenotyping of an AQP2-knockin mouse model (12). This finding could also be supported by the observation that mice deficient in the basolateral water channel AQP3 were able to promptly generate partially concentrated urine in response to dDAVP (1-desamino-8-d-arginine-vasopressin) administration or thirsting before changes in apical water channel AQP2 expression occurred (10). Yang et al. (12) generated a knockin AQP2-T126M mouse model for recessive nephrogenic diabetes insipidus by targeted gene replacement. AQP2-T126M point mutation results in non-X-linked recessive nephrogenic diabetes insipidus in human patients because it causes misfolding of the AQP2 channel, retention in the endoplasmic reticulum, and rapid degradation in AQP2-T126M-transfected mammalian cells (13). Both the AQP2-total-KO in this study and the knockin AQP2-T126M mice in a previous study (12) showed no weight difference at birth compared with wild-type mice, but they failed to thrive and died within 2 weeks of birth. Morphological examination of the kidneys also revealed CD dilatation and medullary atrophy, indicating either very severe impairment of the postnatal kidney development or changes induced by the extreme urine production. Previous light microscopic examination demonstrated no gross morphological differences in the kidneys of basolateral water channel AQP3- or AQP4-null mice as compared with the wild type (9, 10). The observed changes of kidney structures in AQP2-deficient mice should be studied in the future.
AQP2-CD-KO Mice.
By using the Hoxb7-Cre mice expressing the Cre recombinase exclusively in the CD, we were able to produce tissue-specific AQP2-CD-KO mice with sustained AQP2 expression in the CNT and an almost total lack of functional AQP2 in the CD. The efficiency of the Cre recombination in Hoxb7-Cre mice was consistent with results obtained in a previous study (14), in which, in adult mice, less than 1.5% of the cells in the CDs showed immunostaining of the targeted epithelial sodium channel α-subunit (αENaC), indicating that the recombination efficiency using Hoxb7-Cre is close to 100%. In contrast to AQP2-total-KO mice, the AQP2-CD-KO mice survived to adulthood. However, they showed a 10-fold increase in urine output and a markedly decreased urinary osmolality compared with control (Cre−) mice. Moreover, 3-h water deprivation of AQP2-CD-KO mice caused no or only a marginal decrease in urine output compared with the 3-h control period with free access to water. The minor reduction in urine output is not likely to be due to the restoration of urinary concentrating ability but is more likely due to the decreased renal plasma flow and decreased glomerular filtration rate caused by contraction of extracellular fluid volume. Consistent with this interpretation, the osmolality of the urine excreted during the 3-h dehydration period was not different from the control period with free access to water. These results present direct evidence for a profound role for the CD in urinary concentration and body water balance. Interestingly, in contrast to the importance of CD function in water balance regulation presented in this study, a recent study demonstrated that selective deletion of αENaC, an apical sodium channel, in the cortical CD of mice did not result in sodium loss, and the knockout mice were resistant to sodium restriction challenge, suggesting that only the CNT and late distal convoluted tubule are quantitatively important in maintaining sodium balance (14).
It may have been predicted that the Hoxb7-targeted knockout would have resulted in a phenotype in which the kidney behaves like an amphibian kidney in response to antidiuretic hormones. This view is based on the classic observations of Gottschalk (15) and Wirz (16) in rodents showing that, during antidiuresis, osmotic equilibration with the cortical interstitium occurs in the micropuncturable cortical distal tubule. There was no equilibration in the “early distal tubule” (the distal convoluted tubule), but equilibration did occur in the “late distal tubule” (largely made up of the CNT). Thus, it would be expected that, in the Hoxb7-directed knockout, osmotic equilibration would still occur in the cortex, as it does in amphibia. This assumption is contrary to the osmolalities reported in Fig. 8 showing that after 3-h water restriction (with presumably high vasopressin levels), the urinary osmolality did not rise above 200 milliosmoles/kg of H2O. This finding implies that osmotic equilibration did not occur in the cortex and suggests that the cortical parts of the CD system (cortical CD and initial CT) play a necessary role in cortical osmotic equilibration.
The role of AQP2 in the CNT has been undefined, and the function of the CNT in water balance regulation is poorly understood because it is not accessible by either micropuncture or in vitro microdissection techniques. Kishore et al. (17) examined the expression of AQP2 and V2-receptor in renal arcades from normal rat kidney, which connect deep- and mid-cortical nephrons to the cortical CD in the renal cortex and are composed of 70–80% CNT cells and 20–30% intercalated cells. In contrast to Imai's observation (18) that microperfused rabbit kidney arcade segments had a low basal water permeability that was insensitive to vasopressin exposure, they found that vasopressin-regulated AQP2 was highly expressed in the arcades of rat kidney and that the expression of AQP2 was significantly increased in response to water deprivation for 48 h (17). This finding indicates that the CNT segment also plays a vital role in the vasopressin-mediated water reabsorption through AQP2 regulation in rat kidney. In the present study, we examined the role of AQP2 expression in both the CNT and CD in kidneys of control mice by direct comparison with AQP2-total-KO and AQP2-CD-KO mice. The results suggest that AQP2 expression in the CNT is essential for water reabsorption in this segment and plays an essential role in rescuing the lethal phenotype seen in AQP2-total-KO mice. However, it should be mentioned that the residual AQP2 expression in a few CD cells in AQP2-CD-KO mice is a result of incomplete Cre recombination. Although this hypothetically may improve the severe phenotype of the pups and allow survival past the weaning period, the very low level of frequency of the AQP2-positive cells makes this highly unlikely. Immunohistochemistry revealed that the labeling intensity of AQP2 in the CNT of AQP2-CD-KO and control mice was similar, despite the presumed high plasma vasopressin level in the AQP2-CD-KO mice. Moreover, depriving AQP2-CD-KO mice of water for 3 h (likely to be associated with a very high plasma vasopressin levels) failed to increase their urine osmolality, suggesting that vasopressin-regulated water reabsorption though AQP2 present in the CNT is minimal. Finally, an alternative (although unlikely) explanation for the inadequate ability of the AQP2-CD-KO mice to equilibrate the proto-urine with the cortical interstitium could be that salt reabsorption in the CD redilutes the urine. However, the sodium-handling capacity of the CD is too low for this explanation to be of major importance.
Materials and Methods
Generation of the AQP2flx/flx Transgenic Mice.
The targeting construct for creating the floxed AQP2 mutation was produced by transferring a 9.5-kb fragment spanning AQP2 gene exons 1–4 from the bacterial artificial chromosome clone 288-C3 into a truncated pBluescript II (Stratagene) vector by homologous recombination in Escherichia coli strain DY380. The loxP site was introduced into intron 3. Two segments of intron 3 were amplified by PCR, the loxP and BamHI sites were added at the end of the first PCR product, and the BamHI site was added at the end of the second PCR product. The two PCR products were digested with BamHI and ligated, restoring intron 3 with the loxP site inserted in the middle. This fragment was digested with SbfI and BclI and replaced the SbfI–BclI fragment of intron 3 in the construct by directional cloning. The neomycin phosphotransferase expression cassette flanked by two loxP sites was inserted into intron 2 by homologous recombination in E. coli and selection for kanamycin resistance. Finally, a thymidine kinase expression cassette was introduced to the NotI site in pBluescript II backbone. The targeting construct was linearized and electroporated into ES cells derived from 129/Sv mice (19). G418-resistant colonies were selected and expanded. The clones with homologous recombination were detected by Southern blots using probes flanking the targeting construct sequence (probe 1 and probe 2). The neomycin phosphotransferase expression cassette was deleted from the floxed AQP2 allele by transient transfection with Cre-expression plasmid. The neomycin-sensitive clones were screened by PCR for the presence of the correct loxP recombination event, and candidate clones were confirmed by Southern blots (probe 3).
Chimeric mice were generated by injection of the ES cells into B6D2F2 mouse blastocysts. The chimeric males were bred with C57BL/6 females. The agouti offspring (indicating germ-line transmission of the manipulated 129/Sv ES cells) was tested for the presence of AQP2flx mutation by PCR using genomic tail DNA and the primer pair 5′-CTCCATGAATCCAGCCCGCTCC and 5′-ATATCATTTACTGGGTTCTG. The expected product for the wild-type allele is 334 bp and for the AQP2flx allele it is 359 bp (+loxP). The heterozygous mice were further bred to obtain homozygous AQP2flx/flx mice (Fig. 1). That the sequence of the loxP sites was correct was confirmed by sequencing.
Generation of AQP2-Total-KO.
AQP2-total-KO mice were obtained by breeding the AQP2flx/flx mice with the total deletor EIIa-Cre strain mice (developed by H. Westphal, National Institutes of Health, Bethesda). In the offspring, the early embryonic expression of Cre recombinase causes removal of AQP2 exon 3 in the AQP2flx allele (the resulting allele was named AQP2ΔE3) in all mouse organs and germ line. The inheritance of the Cre recombinase gene in the offspring was detected by PCR using genomic tail DNA and the primer pair 5′-GCGGTCTGGCAGTAAAAACTATC and 5′-GTGAAACAGCATTGCTGTCACTT. The disruption of the AQP2flx allele was confirmed by PCR using primer pair 5′-GGTTTCAGCTGCCCTACAAG and 5′-ATATCATTTACTGGGTTCTG. Breeding of the AQP2+/ΔE3EIIa-Cre+ mice with the AQP2flx/flx mice produced AQP2ΔE3/ΔE3EIIa-Cre+ knockout offspring and AQP2flx/ΔE3EIIa-Cre− controls.
Conditional Knockout of AQP2 Gene in the CD.
CD-specific AQP2 gene disruption was obtained by breeding AQP2flx/flx mice with a transgenic line expressing Cre recombinase under the Hoxb7 promoter, Hoxb7-Cre mice (8), and Cre recombinase expression pattern has been characterized in detail in ref. 14. Eight- to 12-week-old AQP2flx/flxHoxb7-Cre+ mice were used, and AQP2flx/flxHoxb7-Cre− siblings served as controls.
Urine and Blood/Serum Measurements.
Twenty-four-hour urine samples were obtained by placing the mice in specially designed minimetabolic cages. Three-hour urine samples were obtained from mice with no access to the water (1000–1300 hours). Control 3-h urine samples were obtained from the same mice on a subsequent day when mice had free access to water. While the mice were under isoflurane anesthesia, blood samples were obtained from the heart right ventricle. Plasma and urine sodium, creatinine, and osmolality were determined.
Immunohistochemistry.
The kidneys were immersion-fixed for 24 h (AQP2-total-KO) or perfusion-fixed and post-fixed for 1 h (AQP2-CD-KO) using 3% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4), embedded in paraffin, and processed for immunohistochemistry as described in ref. 20 by using previously characterized anti-AQP2 antibodies.
Protein Sample Preparation and SDS/PAGE and Immunoblotting.
The inner medulla was homogenized and the homogenate was centrifuged at 4,000 × g for 15 min at 4°C, the supernatant was used for immunoblotting or centrifuged at 17,000 × g for 1 h, and the pellet was used for immunoblotting (20). Samples were loaded on 9% or 12% polyacrylamide gels, electroeluted to nitrocellulose membranes, and subjected to immunolabeling (20).
Acknowledgments
We thank Helle Høyer, Lotte Vallentin Holbech, Ida Maria Jalk, Peter M. Kragh, Inger Merete Paulsen, Zhila Nikrozi, and Mette Vistisen for expert technical assistance and Annette C. Füchtbauer for advice and technical assistance. This work was supported by the Danish National Research Foundation, the Karen Elise Jensen Foundation, Human Frontier Science Program, Danish Medical Research Council, European Commission Grants QRLT 2000 00778 and QRLT 2000 00987, and Regional Technology Innovation Program of the Ministry of Commerce, Industry, and Energy (Korea) Grant RTI04-01-01 (to T.-H.K.).
Abbreviations
- AQP
aquaporin
- CNT
kidney connecting tubule
- CD
collecting duct.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Nielsen S., DiGiovanni S. R., Christensen E. I., Knepper M. A., Harris H. W. Proc. Natl. Acad. Sci. USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S., Chou C. L., Marples D., Christensen E. I., Kishore B. K., Knepper M. A. Proc. Natl. Acad. Sci. USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marples D., Knepper M. A., Christensen E. I., Nielsen S. Am. J. Physiol. 1995;269:C655–C664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 4.Sabolic I., Katsura T., Verbavatz J. M., Brown D. J. Membr. Biol. 1995;143:165–175. doi: 10.1007/BF00233445. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T., Sasaki S., Fushimi K., Ishibashi K., Yaoita E., Kawasaki K., Marumo F., Kihara I. Am. J. Physiol. 1995;268:C1546–C1551. doi: 10.1152/ajpcell.1995.268.6.C1546. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S., Frøkiær J., Marples D., Kwon T.-H., Agre P., Knepper M. A. Physiol. Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 7.Christensen B. M., Wang W., Frøkiær J., Nielsen S. Am. J. Physiol. Renal Physiol. 2003;284:F701–F717. doi: 10.1152/ajprenal.00234.2002. [DOI] [PubMed] [Google Scholar]
- 8.Yu J., Carroll T. J., McMahon A. P. Development (Cambridge, U.K.) 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 9.Ma T., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. J. Clin. Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma T., Song Y., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. Proc. Natl. Acad. Sci. USA. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma T., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. J. Biol. Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 12.Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. J. Biol. Chem. 2001;276:2775–2779. doi: 10.1074/jbc.M008216200. [DOI] [PubMed] [Google Scholar]
- 13.Tamarappoo B. K., Verkman A. S. J. Clin. Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubera I., Loffing J., Palmer L. G., Frindt G., Fowler-Jaeger N., Sauter D., Carroll T., McMahon A., Hummler E., Rossier B. C. J. Clin. Invest. 2003;112:554–565. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottschalk C. W., Mylle M. Am. J. Physiol. 1959;196:927–936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- 16.Wirz H. Helv. Physiol. Pharmacol. Acta. 1956;14:353–362. [PubMed] [Google Scholar]
- 17.Kishore B. K., Mandon B., Oza N. B., DiGiovanni S. R., Coleman R. A., Ostrowski N. L., Wade J. B., Knepper M. A. J. Clin. Invest. 1996;97:2763–2771. doi: 10.1172/JCI118731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai M. Kidney Int. 1979;15:346–356. doi: 10.1038/ki.1979.46. [DOI] [PubMed] [Google Scholar]
- 19.Swiatek P. J., Gridley T. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 20.Rojek A., Nielsen J., Brooks H. L., Gong H., Kim Y. H., Kwon T.-H., Frøkiær J., Nielsen S. Am. J. Physiol. 2005;288:F1276–F1289. doi: 10.1152/ajprenal.00305.2004. [DOI] [PubMed] [Google Scholar]