Abstract
Context: Although poor paraspinal muscle endurance has been associated with less quadriceps activation (QA) in persons with a history of low back pain, no authors have addressed the acute neuromuscular response after lumbar paraspinal fatiguing exercise.
Objective: To compare QA after lumbar paraspinal fatiguing exercise in healthy individuals and those with a history of low back pain.
Design: A 2 × 4 repeated-measures, time-series design.
Setting: Exercise and Sport Injury Laboratory.
Patients or Other Participants: Sixteen volunteers participated (9 males, 7 females; 8 controls and 8 with a history of low back pain; age = 24.1 ± 3.1 years, height = 173.4 ± 7.1 cm, mass = 72.4 ± 12.1 kg).
Intervention(s): Subjects performed 3 sets of isometric lumbar paraspinal fatiguing muscle contractions. Exercise sets continued until the desired shift in lumbar paraspinal electromyographic median power frequency was observed. Baseline QA was compared with QA after each exercise set.
Main Outcome Measure(s): An electric burst was superimposed while subjects performed a maximal quadriceps contraction. We used the central activation ratio to calculate QA = (FMVIC/[FMVIC + FBurst])* 100, where F = force and MVIC = maximal voluntary isometric contractions. Quadriceps electromyographic activity was collected at the same time as QA measurements to permit calculation of median frequency during MVIC.
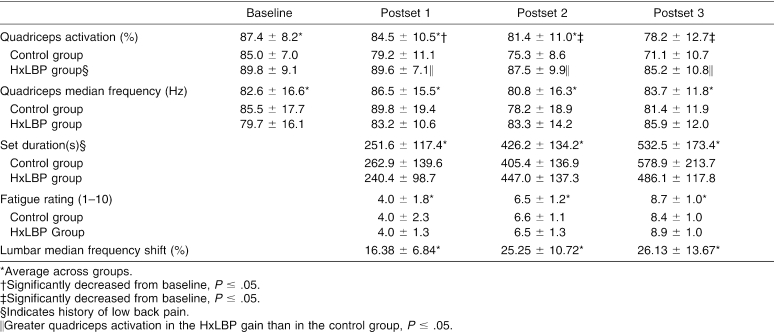
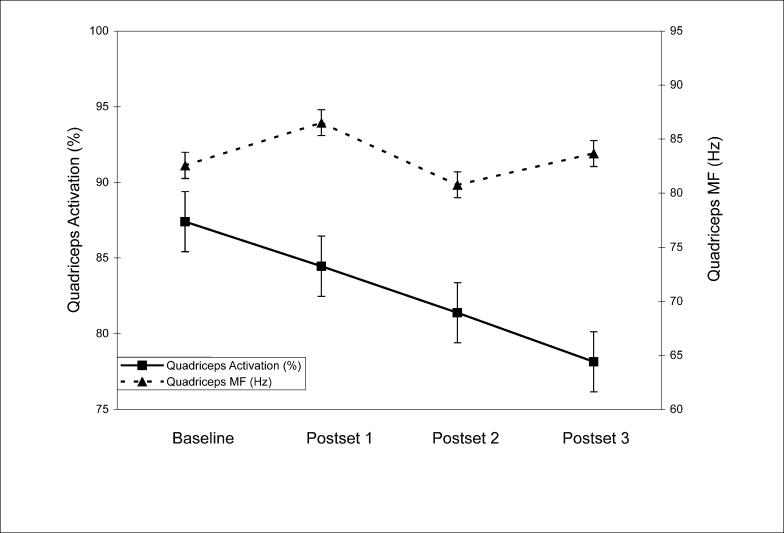
Results: Average QA decreased from baseline (87.4% ± 8.2%) after the first (84.5% ± 10.5%), second (81.4% ± 11.0%), and third (78.2% ± 12.7%) fatiguing exercise sets. On average, the group with a history of low back pain showed significantly more QA than controls. No significant change in quadriceps median frequency was noted during the quadriceps MVICs.
Conclusions: The quadriceps muscle group was inhibited after lumbar paraspinal fatiguing exercise in the absence of quadriceps fatigue. This effect may be different for people with a history of low back pain compared with healthy controls.
Keywords: superimposed burst technique, quadriceps muscle inhibition, low back pain
Decreased force production capability in a muscle is a natural consequence of prolonged, intense muscular exercise.1 The causes of task failure during fatiguing exercise may arise from spinal or supraspinal central factors, such as decreased central drive, or other peripheral factors occurring distal to the neuromuscular junction, such as accumulation of metabolic by-products.2–4 Muscle fatigue can be reliably indexed5,6 as a shift in motor unit recruitment from higher-frequency to lower-frequency motor units, measured with surface electromyography (EMG). Muscle fatigue may contribute to decreased joint stability as a result of impaired joint proprioception7–9 and reduced muscle activation10 and may require neuromuscular compensations in order to maintain normal function during activity.11–14
Persons with low back pain tend to have poor muscular strength and rapid fatigue rates in the lumbar flexors and extensors.15 The onset and progression to fatigue are probably more rapid in muscles that have poor strength and endurance. Therefore, the rate of fatigue in muscles that provide support to the lumbar spine during exercise may be greater in those with low back pain. Persons with low back pain who fatigue more rapidly during isometric lumbar paraspinal exercise have greater amounts of quadriceps inhibition (ie, less quadriceps activation).16 This finding suggests an association between isometric lumbar extension endurance and muscle inhibition but does not address the short-term consequences to lower extremity muscle after lumbar paraspinal muscle fatigue. To date, no authors have measured the acute effects of lumbar paraspinal fatiguing exercise on quadriceps activation.
Muscle inhibition has been described as the diminished ability to contract a muscle voluntarily17 and can result from muscle fatigue10 or simulated joint effusion.18 When a muscle is inhibited, regardless of the cause, it is unable to voluntarily recruit all motor units in its motor neuron pool. Despite previous research findings,19,20 not all mechanisms of muscle inhibition are known. In the lower extremity, quadriceps inhibition commonly follows knee joint injury.17 Insufficient strength due to excessive quadriceps inhibition may alter gait patterns21 or cause compensatory force attenuation strategies during landing22 that may leave lower extremity joints at risk for injury. Comprehending the acute relationship between the lumbar paraspinals and quadriceps may lead to a better understanding of the neuromuscular adaptations that may occur in the lower extremity in response to fatiguing lumbar extension exercise. Therefore, our purpose was to compare quadriceps activation (QA) after isometric lumbar fatiguing exercise in persons with low back pain and healthy individuals with no history of low back pain.
METHODS
A 2 × 4 repeated-measures, time-series design was used with one between factor of group (control, history of low back pain [HxLBP]) and one within factor of time (baseline, postexercise set 1, set 2, and set 3). The independent variables were group (control and HxLBP) and time (baseline, postlumbar paraspinal fatiguing exercise set 1, postset 2, and postset 3). The dependent variables were quadriceps EMG median frequency (MF)23,24 and quadriceps central activation ratio (QA) measured with the superimposed burst technique.25,26
Subjects
Sixteen subjects volunteered for this study (9 males, 7 females; age = 24.1 ± 3.1 years, height = 173.4 ± 7.1 cm, mass = 72.4 ± 12.1 kg) (Table 1). From previous data,16 we determined a priori that 16 subjects were necessary to find differences in QA at an α level of .05 while maintaining statistical power greater than 1 − β = .8, based on the observed group effect comparing quadriceps inhibition between subjects with low back pain and either highly fatigable or less fatigable lumbar paraspinal muscles. Our university's institutional review board approved this study before data collection. All subjects read and signed an informed consent agreement.
Table 1. Subject Demographics (Mean ± SD).
None of the subjects had current low back pain, and none had evidence of sensory, motor, or reflex deficits during the physical examination. Participants also reported no history of cancer, inflammatory or rheumatoid disease (including arthritis), a vertebral disc herniation, vertebral fracture, other neurologic problem in the low back or legs, or knee joint injury. The HxLBP group consisted of subjects who reported any history of low back pain and/or reported having low back pain after exercise. For example, a subject who answered “no” to the question, “Do you have back pain right now?” (Table 2) but reported a duration of low back pain when asked “How long have you had low back pain,” or said “yes” to the question, “Do you have low back pain after you exercise?” was included in the HxLBP group. Subjects in the control group reported no history of low back pain and reported never having had low back pain after exercise. For example, those who answered “no” to questions 1, 3, and 4 and reported zero months of low back pain duration in question 2 were included in the control group.
Table 2. Interview Questions.
Instruments
Load Cell
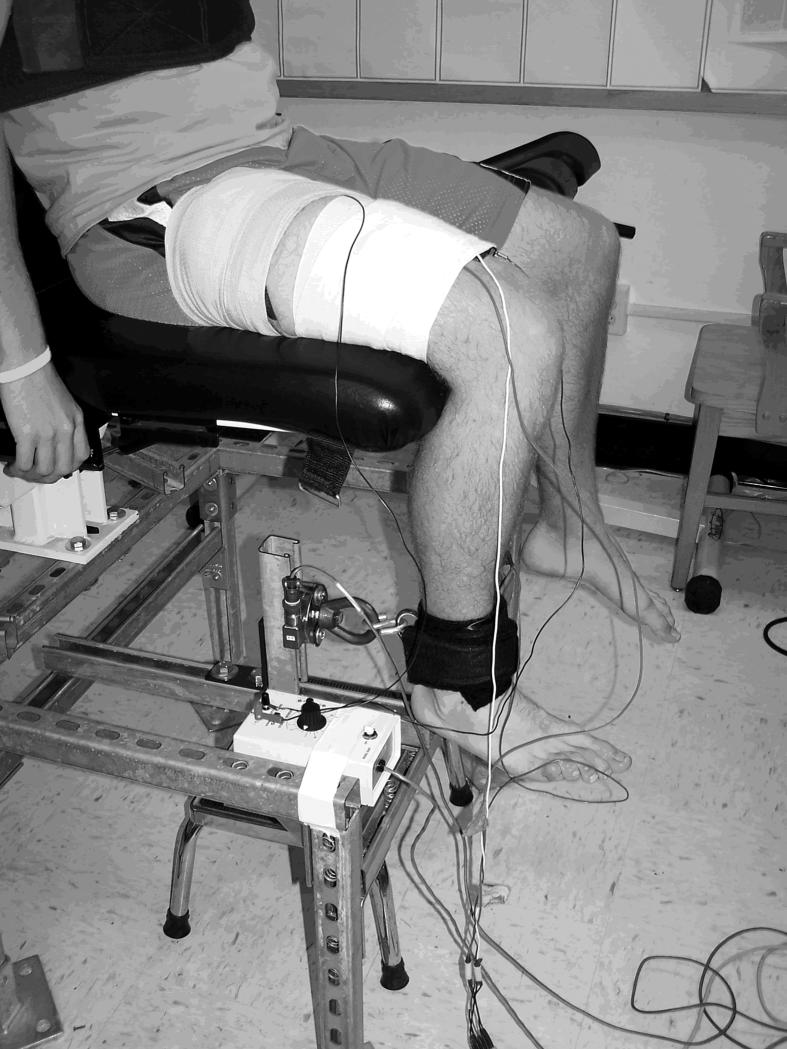
The force of knee extension was measured with a strain gauge pancake load cell (model 41-tension/compression; Honeywell Sensotec, Columbus, OH) mounted to a steel frame beneath a chair (Figure 1). This load cell is capable of measuring up to 500 000 lb (226 796.19 kg) at an infinite resolution. Signals from the load cell were amplified and digitized with a 12-bit data acquisition system at 1000 Hz (model MP100; Biopac Systems, Inc, Goleta, CA) through a universal amplifier (model DA100c).
Figure 1. The experimental setup for simultaneous measurement of quadriceps activation using the superimposed burst technique and quadriceps electromyography.
Surface Electromyography
Electric activity in the lumbar paraspinal and quadriceps muscles was collected with surface EMG. Signals were amplified with a high-gain, differential-input, biopotential amplifier (model EMG100C; Biopac Systems, Inc) with a gain of 1000. Analog signals were digitized with a 12-bit data acquisition system (model MP100; Biopac Systems, Inc) at 2000 Hz with a common mode rejection ratio of 110 dB, an input impedance of 1.0 MΩ, and a noise voltage of 0.2 μV.
Procedures
Before data collection, we screened subjects for group assignment. The objective of the screening was to separate subjects into 2 groups: healthy individuals with no history of low back pain (control) and persons who reported having low back pain in the past (HxLBP). We used a scripted interview (Table 2) and physical examination to screen the subjects. The interview and physical examination were intended to identify persons with a history of low back pain that was not due to disc or bone injury or tumor and who could safely perform the fatiguing exercise sets.
After this interview, a physical examination was performed on all subjects. During the examination, an experienced, licensed certified athletic trainer (J.M.H.) performed a lower extremity dermatome, myotome, and deep tendon reflex (patellar and Achilles) evaluation and a bilateral straight-leg raise test.27 Subjects were also asked to perform active standing lumbar extension. Potential participants were excluded if they displayed any lower quarter neurologic bilateral asymmetry, intolerable pain (>3/10) with standing lumbar extension, the inability to extend the spine at least 15° comfortably, or a positive straight-leg test. The intention of this screening was to identify a group of persons who were at risk for developing low back pain due to poor lumbar muscle strength or endurance by excluding persons with a history of other (nonmuscular) common causes of low back pain. The control group was not a matched cohort to the HxLBP group.
After group assignment, subjects were fit with EMG electrodes and stimulating electrodes. To minimize skin resistance during signal acquisition, the skin was shaved, lightly debrided with fine sandpaper, and cleaned thoroughly with isopropyl alcohol before electrode placement. Self-adhesive, round, small-diameter (35-mm), pre-gelled Ag-AgCl surface electrodes collected signals from muscle groups of interest. We verified appropriate electrode placement over active muscle by palpating the muscle during an active contraction. The quadriceps electrodes were placed over the vastus lateralis, approximately 10 cm proximal to the patellar base. The lumbar paraspinal muscle electrodes were placed at approximately the L4-L5 level over active tissue about 3 to 4 cm lateral to the vertebral spinous processes. Electrodes were placed parallel to muscle fiber orientation, with an interelectrode distance of 2 cm. A ground electrode was placed on the anterior mid tibia. The 2 stimulating electrodes were 8- × 14-cm rubber electrodes coated with aqueous conductive gel and secured to the anterior thigh with a compression wrap. One stimulating electrode was placed at the proximal-lateral thigh and the other at the distal-medial thigh directly over the quadriceps muscle group. Subjects were then secured to the chair in a seated position with the knee bent to 90° for baseline QA measurements.
Quadriceps Activation
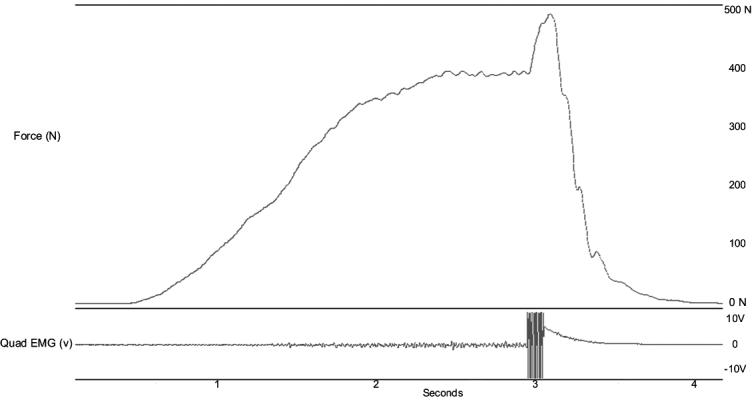
We used the superimposed burst technique25,26 to measure the extent of QA. We measured QA first at baseline and again after each lumbar paraspinal fatiguing exercise set. Subjects sat in a chair mounted to a steel frame and performed a maximal voluntary isometric contraction (MVIC) of the knee extensors. Once the MVIC reached a maximal plateau, a researcher manually triggered an electric stimulus delivered directly to the quadriceps through the stimulating electrodes. The S88 dual-output, square-pulse stimulator with the SIU8T transformer stimulus isolation unit (Grass-Telefactor, West Warwick, RI) was used to manually deliver a 100-millisecond train of 10 square-wave pulses at an intensity of 125 V. Individual pulse duration was 600 microseconds, delivered at a carrier frequency of 100 pulses per second. The stimulus was superimposed while the subjects were maximally contracting the quadriceps. In theory, this stimulus recruited all the remaining motor neurons in the quadriceps motor neuron pool that were not voluntarily recruited during the MVIC, thus causing a transient burst of force over the MVIC force (Figure 2). We determined the mean MVIC force value (volts) for a 150-millisecond period immediately before the stimulation and the peak force value (volts) during the twitch response to the superimposed burst. A ratio between the mean MVIC force (FMVIC) and maximum burst force (FBurst) was used to calculate the percentage of QA where QA = (FMVIC/[FMVIC + FBurst]) * 100. This ratio, called the central activation ratio, has been used previously to describe the extent of QA.25,26 The average of 3 trials was used for calculating QA at each level of time.
Figure 2. Graphs of force (top) and electromyographic activity (bottom). Force (N) and electromyography (V) data were collected simultaneously during the superimposed burst technique. The same 150-millisecond time epoch was used for quadriceps maximal voluntary isometric contraction force and median frequency measurements.
Lumbar Paraspinal Fatiguing Exercise
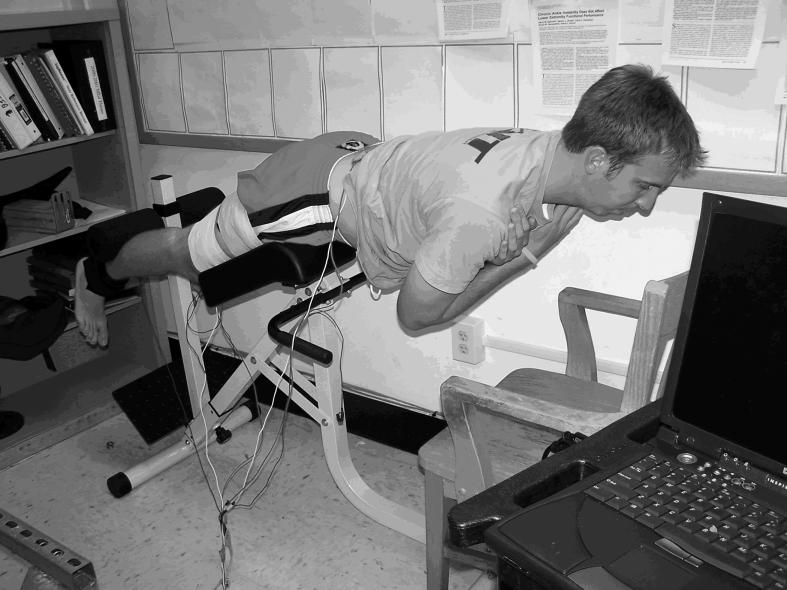
After a baseline measure of QA, subjects performed 3 sets of lumbar fatiguing exercise that consisted of repeated 10-second periods of gravity-resisted isometric contractions followed by a 10-second rest. A lumbar hyperextension chair (Recreation Supply Inc, Sunbury, OH) allowed subjects to comfortably perform isometric lumbar paraspinal muscle contractions. Subjects were positioned so their anterior-superior iliac spines were not in contact with the hip pad. The legs were positioned under leg pads for leverage (Figure 3). A wooden desk chair was placed in front of the lumbar hyperextension device so that subjects could rest comfortably between fatiguing repetitions. During the isometric contractions, subjects were verbally encouraged to maintain a trunk position parallel to the floor. A 2-second EMG sample was recorded during the first repetition of the first set. Using macro software (Macro-Magic, Iolo Technologies, LLC, Los Angeles, CA), we recorded the steps necessary to calculate the MF of the recorded EMG.28 For frequency analysis, raw signals were decomposed into the frequency domain through the fast Fourier transform algorithm, using a Hamming window, for analysis. The number of data points selected for the fast Fourier tranformation must be a power of 2 in order to increase the frequency resolution and expedite the decomposition calculations. Therefore, we substituted, or padded, the 3990 data points (approximately 2 seconds × 2000 Hz) selected from the lumbar paraspinal EMG signals with 106 zeros. Similarly, we padded the 301 data points (approximately 150 milliseconds × 2000 Hz) selected from the quadriceps EMG signal with 211 zeros.
Figure 3. The experimental setup for performing lumbar paraspinal muscle isometric contractions (figure is of the contracted state).
We played the prerecorded MF calculation procedure at 500 times speed in order to quickly return the MF as close to “real time” as possible. Typically, we were able to return the MF for the desired time epoch in 15 to 20 seconds. This allowed us to record the MF from all repetitions. As the subjects' lumbar paraspinal muscles fatigued, we monitored the downward shift in MF, which represented a shift in motor unit recruitment from fast twitch to slow twitch fibers.6 We used the percentage shift in MF as a marker of muscle fatigue during the lumbar extension exercise. The highest MF recorded during the first exercise set served as a baseline measurement and represented the frequency content of the paraspinal muscles in an unfatigued state.
The MF was recorded for each repetition of the first exercise set until a 10% shift was observed. For example, if the baseline MF was 100 Hz, then the subject was instructed to stop once the recorded MF during the exercise set reached 90 Hz. For the second exercise set, the subject was instructed to stop once the recorded MF dropped to 25% below the baseline established during the first set. The third exercise set was stopped once the recorded MF shifted to 50% below the baseline established during the first set. Exercise sets were stopped if a subject was unable to continue due to intolerable fatigue or pain in the lumbar or sacroiliac area. We chose these percentages from pilot data in order to establish MF shifts that would correspond with increasing levels of fatigue. We were able to approximately reach the goals for the first and second sets; however, several subjects were unable to continue due to extreme muscle fatigue during the third set before a 50% shift was observed (Table 3).
Table 3. Quadriceps Activation, Quadriceps Median Frequency, Set Duration, Fatigue Rating, and Lumbar Median Frequency Shift (Mean ± SD).
For descriptive purposes, the duration of each set was timed with a stopwatch, and each subject provided a “perceived fatigue” rating that ranged from 0 to 10 after each exercise set. A rating of 0 described an unfatigued state, and 10 represented complete muscle failure due to fatigue.
Quadriceps Electromyography
Vastus lateralis EMG was recorded while the subject was performing an MVIC during the QA measurements. In order to determine whether subjects were experiencing quadriceps muscle fatigue due to repeated maximal contractions, we calculated MF of the quadriceps post hoc. The procedures to calculate quadriceps and lumbar paraspinal MF were similar. However, for the quadriceps MF calculation, we used a time epoch of 150 milliseconds, time matched to the epoch used to calculate mean force during the MVIC. The average MF at each level of time was used for analysis. Average quadriceps MF was compared at each level of time in order to identify a significant change in motor unit recruitment, possibly due to quadriceps fatigue from repeated maximal contractions.
Statistical Analysis
We calculated a 2 × 4 (group × time) analysis of variance with repeated measures on time to determine if QA was different over time and between groups. We also performed a 1-way analysis of variance with repeated measures to determine mean differences in quadriceps MF at each level of time. Follow-up simple contrasts (univariate F tests) were conducted to compare postfatiguing exercise QA with baseline QA if necessary. We also performed planned comparisons between groups by using univariate F tests for QA at each level of time. The SPSS statistical package (version 11.0; SPSS Inc, Chicago, IL) was used for all statistical analyses. An α level of P ≤ .05 was identified a priori for all statistical tests.
RESULTS
No significant group × time interaction (F3,42 = 2.49, P = .07, η2 = .15, 1 − β = .58) was noted. A main effect for time (F3,42 = 9.70, P < .001, η2 = .41, 1 − β = 0.99) suggested differences in QA over time (see Table 3). Simple contrasts showed that QA was significantly reduced from baseline after the first (P = .051), second (P < .001), and third (P = .001) lumbar paraspinal exercise sets. A main effect for group (F1,14 = 6.25, P = .03, η2 = .31, 1 − β = .64) indicated that the HxLBP group had higher average QA (88.1 ± 9.2) than controls (77.7 ± 9.4). The HxLBP group exhibited significantly higher QA after the first (F1,15 = 1.39, P = .04), second (F1,15 = 4.95, P = .02), and third (F1,15 = 6.98, P = .02) exercise sets but not at baseline (F1,15 = 1.39, P = .26). Finally, no significant change was noted in quadriceps MF over time (F3,42 = 2.19, P = .10, η2 = .14, 1 − β = .52).
DISCUSSION
Quadriceps activation was reduced with fatiguing exercise to the lumbar paraspinal muscles, even though the quadriceps were not fatigued (Figure 4). Overall spinal stability involves contributions from multiple muscles and muscle groups surrounding the lumbar spine.29 Leetun et al30 discussed the role of pelvic and trunk stabilization on control of lower extremity movements. In their study, basketball and track athletes who did not sustain lower extremity injuries had stronger hip abduction and greater external rotation strength.30 Although the authors found no association between trunk extensor endurance and the risk of lower extremity injury, other results16,31–33 suggest a connection between the muscles that stabilize the spine and those that stabilize the lower extremity. First, Hodges and Richardson33 reported that trunk muscles that contribute to spinal stability activate before lower extremity movements. This suggests that the body attempts to stabilize the spine as a foundation for lower extremity movements. In addition, Suter and Lindsay16 reported reduced QA in subjects with a history of low back pain whose lumbar paraspinal muscles were quicker to fatigue during an extended isometric contraction. Finally, conservative treatment for low back dysfunction including sacroiliac joint manipulations caused quadriceps disinhibition in persons with symptomatic anterior knee pain.31,32
Figure 4. Quadriceps activation and quadriceps median frequency (MF) measured simultaneously over time (±SEM).
From our data, we cannot determine whether the reduced QA would be meaningful in an active setting. Quadriceps inhibition (ie, reduced QA) can result in kinematic and kinetic changes during gait.21,34,35 This may compromise the ability of the lower extremity muscles to appropriately respond to joint loading, resulting in changes in gait mechanics. Quadriceps inhibition after anterior cruciate ligament injury36 causes adaptations in gait mechanics.34,35,37 The term “quadriceps-avoidance gait” has been used to describe these detrimental gait adaptations that are commonly associated with quadriceps inhibition, which may have long-term adverse effects.34 Future investigators may help us to understand the meaningfulness of the observed QA reduction after fatiguing lumbar extension exercise during gait.
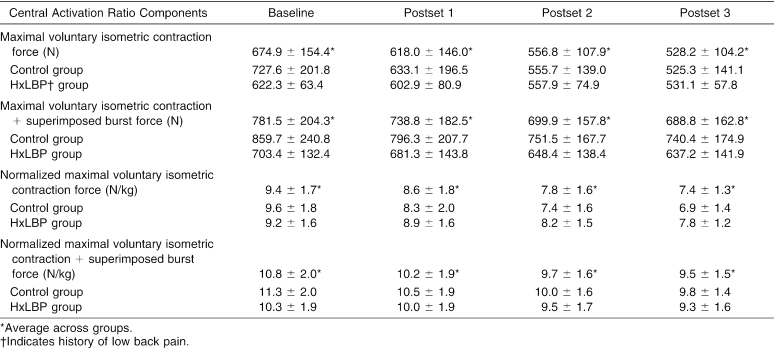
When a muscle is fatigued, fewer motor units are available to call on during muscle contractions.10 Lumbar muscle fatigue causes biomechanical adaptations during lifting tasks38 and reduces trunk proprioception.8 The fatiguing exercise in our study targeted the lumbar extensors; however, we do not know the rate or extent to which each muscle group in the lower extremity and trunk fatigued during the exercise sets. It is probable that some degree of hamstring and gluteal muscle activation occurred during isometric trunk extension. Hamstring and gluteal fatigue contribute to task failure during isometric trunk extension.39 In the present study, we know that the quadriceps were not fatigued, but we did not measure or control for hamstring or gluteal fatigue during the exercise sets. Future investigators should determine muscular contributors other than the lumbar extensors to QA reductions during isometric trunk extension. In addition, we cannot attribute the change in QA to a peripheral or central mechanism of lumbar paraspinal fatigue. We cannot discount the possibility that changes in QA may have central and peripheral origins that may have neuromuscular or metabolic consequences not explained by the present data. However, review of quadriceps MF and individual force components of the central activation ratio leads us to conclude that peripheral quadriceps fatigue from repeated quadriceps MVIC was probably not a factor in the observed inhibition after lumbar extension exercise (Table 4).
Table 4. Average Force Components of Central Activation Ratio Across Group and Time (Mean ± SD).
Subjects in the HxLBP group exhibited, on average, greater QA than controls. We found no difference in baseline QA between the HxLBP group and the control group. This finding is consistent with that of previous authors,16 who reported no differences in quadriceps inhibition between persons with HxLBP and controls. Our findings were similar despite the fact that the subjects in the previous study were considerably heavier (85.5 ± 17.3 kg) and older (37.4 ± 9.9 years) than those in our study (72.4 ± 12.1 kg and 24.1 ± 3.1 years, respectively). However, subjects in the HxLBP group exhibited greater QA after the fatiguing lumbar extension exercise sets. The small sample size and homogeneity of the subjects in each group may have affected this interaction because the subjects in the HxLBP and control groups did not differ considerably other than in the self-reported history of low back pain. All subjects were otherwise healthy, active individuals who would be expected to have similar QA. The possibility of poor endurance or strength in the spine and pelvis stabilization muscles in the HxLBP group may have resulted in a different strategy to compensate for lumbar paraspinal muscle fatigue. However, we cannot be absolutely certain that we ruled out all bone, disc, nerve, and tumor conditions with our interview and physical examination, nor can we be certain that subjects had poor core muscle strength and endurance compared with the control group, as this was not a part of our physical examination or inclusion criteria.
Although multiple factors may contribute to the different mechanisms by which the 2 groups in this study responded to the fatiguing lumbar extension exercise, we offer 2 possible explanations. The first is based on muscle fatigue. Force production capability most likely was reduced as the lumbar paraspinal muscles fatigued during the trunk extension exercise. Subjects with HxLBP may have compensated for lost lumbar stability due to lumbar fatigue by protecting the quadriceps motor neuron pool in order to maintain anterior-posterior symmetry. This would be the most likely explanation given the fact that the quadriceps did not fatigue but were inhibited. In addition, we examined the individual components of the central activation ratio between groups (Table 4) and noticed smaller average superimposed burst force in the subjects with HxLBP than in control subjects. This finding suggests that the subjects with HxLBP may have been recruiting quadriceps motor units from a smaller total motor neuron pool. In the absence of quadriceps fatigue, persons with HxLBP may have fewer quadriceps motor units to be inhibited per kilogram of body mass. Therefore, a relationship between the trunk and knee extensors may exist in persons with poor lumbar muscle stability, perhaps as a protective mechanism to preserve knee function during activity.
Another possible explanation is based on the reciprocal relationship between agonist and antagonist muscles. Subjects in the HxLBP group may have had less strength and endurance in the abdominal muscles. As the lumbar paraspinal muscles fatigued and most likely became inhibited,10 the remaining stabilizing muscles should have responded by co-contracting to protect the lumbar spine. In participants with HxLBP, less abdominal strength may place more of a demand on other muscles, such as the quadriceps, to promote stability and proper function at the pelvis and spine.40 Higher QA in subjects with HxLBP after the fatiguing exercise may be a protective response to compensate for reduced lumbar stability. If the lumbar paraspinal muscles were inhibited due to fatigue while the quadriceps muscles were inhibited without fatigue, from a reciprocal inhibition perspective, one would expect simultaneous hamstring and abdominal activation to maintain anterior-posterior muscle balance for trunk and pelvis stability. If persons with HxLBP adapt to lumbar fatiguing exercise by protecting the quadriceps motor neuron pool, possibly because of weak core-stabilizing muscles, it is necessary to determine how the other muscles, such as the hamstrings, respond and/ or contribute to this relationship. Further, we do not know whether this relationship may be more pronounced in subjects with active low back pain.
Quadriceps fatigue is an inherent limitation when using the superimposed burst technique in a repeated-measures design. In our study, subjects performed 12 maximal quadriceps contractions, each lasting 3 to 5 seconds. Reduced force production by a muscle group is a direct result of local muscle fatigue; however, the central activation ratio assumes this reduction in force is due to a central drive failure. Therefore, it is important to include a measure of fatigue when using this technique for estimating muscle activation. In our study, QA calculations were performed using MVIC and MVIC + superimposed burst force data from each level of time when the subsequent ratios indicated a reduction in QA relative to what was electrically recruitable at each postexercise measure. However, the reduction in MVIC + superimposed burst force seen over time (see Table 4) may be interpreted as local quadriceps fatigue, which may be contributing to the decline in QA after lumbar paraspinal exercise. Although this is contradictory to our quadriceps MF data indicating no quadriceps fatigue, we cannot completely rule this out as a contributing factor to QA reduction.
In conclusion, regardless of group, lumbar paraspinal fatiguing exercise reduced QA. The relationship between the lumbar paraspinal and quadriceps muscles may exist to facilitate stability and potentially maintain normal QA during prolonged activities in persons with HxLBP. The mechanism of the association between lumbar paraspinal fatigue and QA requires further research to investigate interactions among muscles in the lower extremity and core during fatiguing exercise. In addition, future investigators should focus on how the observed QA reduction after fatiguing exercises affects gait in patient populations.
REFERENCES
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Nordlund MM, Thorstensson A, Cresswell AG. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol. 2004;96:218–225. doi: 10.1152/japplphysiol.00650.2003. [DOI] [PubMed] [Google Scholar]
- Schillings ML, Hoefsloot W, Stegeman DF, Zwarts MJ. Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur J Appl Physiol. 2003;90:562–568. doi: 10.1007/s00421-003-0913-4. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol. 1999;80:57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- Ebenbichler GR, Bonato P, Roy SH. Reliability of EMG time-frequency measures of fatigue during repetitive lifting. Med Sci Sports Exerc. 2002;34:1316–1323. doi: 10.1097/00005768-200208000-00013. et al. [DOI] [PubMed] [Google Scholar]
- Dedering A, Nemeth G, Harms-Ringdahl K. Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clin Biomech (Bristol, Avon) 1999;14:103–111. doi: 10.1016/s0268-0033(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Lee HM, Liau JJ, Cheng CK, Tan CM, Shih JT. Evaluation of shoulder proprioception following muscle fatigue. Clin Biomech (Bristol, Avon) 2003;18:843–847. doi: 10.1016/s0268-0033(03)00151-7. [DOI] [PubMed] [Google Scholar]
- Taimela S, Kankaanpaa M, Luoto S. The effect of lumbar fatigue on the ability to sense a change in lumbar position: a controlled study. Spine. 1999;24:1322–1327. doi: 10.1097/00007632-199907010-00009. [DOI] [PubMed] [Google Scholar]
- Lattanzio PJ, Petrella RJ, Sproule JR, Fowler PJ. Effects of fatigue on knee proprioception. Clin J Sport Med. 1997;7:22–27. doi: 10.1097/00042752-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Walton DM, Kuchinad RA, Ivanova TD, Garland SJ. Reflex inhibition during muscle fatigue in endurance-trained and sedentary individuals. Eur J Appl Physiol. 2002;87:462–468. doi: 10.1007/s00421-002-0670-9. [DOI] [PubMed] [Google Scholar]
- Madigan ML, Pidcoe PE. Changes in landing biomechanics during a fatiguing landing activity. J Electromyogr Kinesiol. 2003;13:491–498. doi: 10.1016/s1050-6411(03)00037-3. [DOI] [PubMed] [Google Scholar]
- Nyland JA, Shapiro R, Caborn DN, Nitz AJ, Malone TR. The effect of quadriceps femoris, hamstring, and placebo eccentric fatigue on knee and ankle dynamics during crossover cutting. J Orthop Sports Phys Ther. 1997;25:171–184. doi: 10.2519/jospt.1997.25.3.171. [DOI] [PubMed] [Google Scholar]
- Nyland JA, Caborn DNM, Shapiro R, Johnson DL. Crossover cutting during hamstring fatigue produces transverse plane knee control deficits. J Athl Train. 1999;34:137–143. [PMC free article] [PubMed] [Google Scholar]
- Nyland JA, Caborn DNM, Shapiro R, Johnson DL. Fatigue after eccentric quadriceps femoris work produces earlier gastrocnemius and delayed quadriceps activation during crossover cutting among normal athletic women. Knee Surg Sports Traumatol Arthrosc. 1997;5:162–167. doi: 10.1007/s001670050045. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M, Taimela S, Laaksonen D, Hanninen O, Airaksinen O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil. 1998;79:412–417. doi: 10.1016/s0003-9993(98)90142-3. [DOI] [PubMed] [Google Scholar]
- Suter E, Lindsay D. Back muscle fatigability is associated with knee extensor inhibition in subjects with low back pain. Spine. 2001;26:E361–366. doi: 10.1097/00007632-200108150-00013. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9:135–159. [Google Scholar]
- Palmieri RM, Ingersoll CD, Hoffman MA. Arthrogenic muscle response to a simulated ankle joint effusion. Br J Sports Med. 2004;38:26–30. doi: 10.1136/bjsm.2002.001677. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri RM, Tom JA, Edwards JE. Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol. 2004;14:631–640. doi: 10.1016/j.jelekin.2004.06.002. et al. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Weltman A, Tom JA. An experimental knee joint effusion does not affect plasma catecholamine concentration in humans. Neurosci Lett. 2004;366:76–79. doi: 10.1016/j.neulet.2004.05.016. et al. [DOI] [PubMed] [Google Scholar]
- Torry MR, Decker MJ, Ellis HB, Shelburne KB, Sterett WI, Steadman JR. Mechanisms of compensating for anterior cruciate ligament deficiency during gait. Med Sci Sports Exerc. 2004;36:1403–1412. doi: 10.1249/01.mss.0000135797.09291.71. [DOI] [PubMed] [Google Scholar]
- Ernst GP, Saliba E, Diduch DR, Hurwitz SR, Ball DW. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000;80:251–260. [PubMed] [Google Scholar]
- Mathur S, Eng JJ, MacIntyre DL. Reliability of surface EMG during sustained contractions of the quadriceps. J Electromyogr Kinesiol. 2005;15:102–110. doi: 10.1016/j.jelekin.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Schindler-Ivens S, Williams DM, Chandran R, Sharma SS. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. J Electromyogr Kinesiol. 2003;13:83–92. doi: 10.1016/s1050-6411(02)00050-0. [DOI] [PubMed] [Google Scholar]
- Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Magee DJ. Orthopedic Physical Assessment. 3rd ed. Philadelphia, PA: WB Saunders; 1997.
- EMG Signal Analysis. Biopac, Inc. Available at: http://biopac.com/AppNotes/app118EMG/emg.html. Accessed February 2004.
- Cholewicki J. VanVliet JJt. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin Biomech (Bristol, Avon) 2002;17:99–105. doi: 10.1016/s0268-0033(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Leetun DT, Ireland ML, Willson JD, Ballantyne BT, Davis IM. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc. 2004;36:926–934. doi: 10.1249/01.mss.0000128145.75199.c3. [DOI] [PubMed] [Google Scholar]
- Suter E, McMorland G, Herzog W, Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22:149–153. doi: 10.1016/S0161-4754(99)70128-4. [DOI] [PubMed] [Google Scholar]
- Suter E, McMorland G, Herzog W, Bray R. Conservative lower back treatment reduces inhibition in knee-extensor muscles: a randomized controlled trial. J Manipulative Physiol Ther. 2000;23:76–80. [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77:132–144. doi: 10.1093/ptj/77.2.132. [DOI] [PubMed] [Google Scholar]
- Torry MR, Decker MJ, Viola RW, O'Connor DD, Steadman JR. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000;15:147–159. doi: 10.1016/s0268-0033(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Knoll Z, Kiss RM, Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol. 2004;14:287–294. doi: 10.1016/j.jelekin.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Suter E, Herzog W, Bray R. Quadriceps activation during knee extension exercises in patients with ACL pathologies. J Appl Biomech. 2001;17:87–102. [Google Scholar]
- Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech (Bristol, Avon) 2004;19:136–144. doi: 10.1016/j.clinbiomech.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Bonato P, Ebenbichler GR, Roy SH. Muscle fatigue and fatigue-related biomechanical changes during a cyclic lifting task. Spine. 2003;28:1810–1820. doi: 10.1097/01.BRS.0000087500.70575.45. et al. [DOI] [PubMed] [Google Scholar]
- Plamondon A, Trimble K, Lariviere C, Desjardins P. Back muscle fatigue during intermittent prone back extension exercise. Scand J Med Sci Sports. 2004;14:221–230. doi: 10.1111/j.1600-0838.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- Trafimow JH, Schipplein OD, Novak GJ, Andersson GB. The effects of quadriceps fatigue on the technique of lifting. Spine. 1993;18:364–367. doi: 10.1097/00007632-199303000-00011. [DOI] [PubMed] [Google Scholar]