Abstract
Context: Creatine monohydrate (CrM) use is highly prevalent in team sports (eg, football, lacrosse, ice hockey) and by athletes at the high school, college, professional, and recreational levels. Concerns have been raised about whether creatine use is associated with increased cramping, muscle injury, heat intolerance, and risk of dehydration.
Objective: To assess whether 1 week of CrM supplementation would compromise hydration status, alter thermoregulation, or increase the incidence of symptoms of heat illness in dehydrated men performing prolonged exercise in the heat.
Design: Double-blind, randomized, crossover design.
Setting: Human Performance Laboratory.
Patients or Other Participants: Twelve active males, age = 22 ± 1 year, height = 180 ± 3 cm, mass = 78.8 ± 1.2 kg, body fat = 9 ± 1%, V̇o2peak = 50.9 ± 1 ml·kg−1·min−1.
Intervention(s): Subjects consumed 21.6 g·d−1 of CrM or placebo for 7 days, underwent 48 ± 10 days of washout between treatments, and then crossed over to the alternate treatment in the creatine group. On day 7 of each treatment, subjects lost 2% body mass by exercising in 33.5°C and then completed an 80-minute exercise heat-tolerance test (33.5°C ± 0.5°C, relative humidity = 41 ± 12%). The test consisted of four 20-minute sequences of 4 minutes of rest, alternating a 3-minute walk and 1-minute high-intensity run 3 times, and walking for 4 minutes.
Main Outcome Measures: Thermoregulatory, cardiorespiratory, metabolic, urinary, and perceptual responses.
Results: On day 7, body mass had increased 0.88 kg. No interaction or treatment differences for placebo versus CrM during the exercise heat-tolerance test were noted in thermoregulatory (rectal temperature, 39.3 ± 0.4°C versus 39.4 ± 0.4°C) cardiorespiratory (V̇o2, 21.4 ± 2.7 versus 20.0 ± 1.8 ml·kg−1·min−1; heart rate, 192 ± 10 versus 192 ± 11 beats·min−1; mean arterial pressure, 90 ± 9 versus 88 ± 5 mm Hg), metabolic (lactate, 6.7 ± 2.7 versus 7.0 ± 3.0 mmol·L−1), perceptual thirst (thirst, 7 ± 1 versus 7 ± 1; thermal sensation, 8 ± 2 versus 8 ± 1; rating of perceived exertion, 17 ± 3 versus 17 ± 2), plasma glucose (0–20 minutes of exercise heat-tolerance, 6.5 ± 1.2 versus 6.8 ± 0.8 mmol·L−1), plasma (297 ± 5 versus 300 ± 4 mOsm·kg−1) and urine (792 ± 117 versus 651 ± 134 mOsm·kg−1), urine specific gravity (1.025 ± 0.003 versus 1.030 ± 0.005) and urine color (7 ± 1 versus 6 ± 1) measures were increased during CrM. Environmental Symptoms Questionnaire scores were similar between treatments. The levels of dehydration incurred during dehydration and the exercise heat-tolerance test were similar and led to similar cumulative body mass losses (−4.09 ± 0.53 versus −4.38 ± 0.58% body mass).
Conclusions: Short-term CrM supplementation did not increase the incidence of symptoms or compromise hydration status or thermoregulation in dehydrated, trained men exercising in the heat.
Keywords: hydration, thermoregulation, cardiovascular system, metabolism, ergogenic aids
Creatine monohydrate (CrM) loading (eg, 20 g·d−1 for 5 to 7 days) can cause muscle phosphocreatine content to increase by as much as 50%,1–3 toward what appears to be an upper storage limit of 160 mmol·kg−1 dry muscle.4 This increased phosphocreatine content provides more substrate for the phosphagen system and enhances its resynthesis rate during intense muscular contraction.5 Consequently, maximal or near maximal bursts of strength and power, anaerobic performance, and rapid recovery between explosive exercise bouts can be improved.6–10 Not surprisingly, then, CrM use is highly prevalent in team sports such as football, lacrosse, and ice hockey and by high school,11–13 collegiate,14,15 professional,16 and recreational17 athletes.
Concerns have been raised about creatine use, however, because of anecdotal observations of increased cramping, muscle injury, and heat intolerance18 and suggestions that it increases the risk of dehydration via fluid shifts into the intracellular space.3,19 These concerns are especially relevant to exercise performed in conditions that limit heat dissipation, because exercise performance and, more importantly, individual health depend on temperature regulation and hydration state.
Because of these concerns, the American College of Sports Medicine, in recommendations titled “The Physiological and Health Effects of Oral Creatine Supplementation,”20 stated, “…there are concerns about the possibility of altered fluid balance, and impaired sweating and thermoregulation in athletes acutely loading with creatine in settings where there is potential for thermal stress.”20 Further, “…high-dose creatine supplementation (ie, 20 g·d−1) should be avoided during periods of increased thermal stress, such as sports activities performed under high ambient temperature/humidity conditions.”20 However, no scientific evidence is presented to support this recommendation and, to our knowledge, no studies to date support it.
Anecdotal claims of increased incidence of cramps and injury were not supported by results from a 3-year observation of National Collegiate Athletic Association Division IA college football players using CrM during training and competition.21 Moreover, the 4 groups of authors22–25 who examined CrM use during exercise in the heat reported that creatine use either did not alter22 or attenuated23–25 the thermal stress experienced by euhydrated individuals. Specifically, creatine resulted in decreased heart rate,24,26 a decreased rise in rectal temperature during exercise,24–26 and decreased metabolic rate.24 Indeed, the osmotic property of creatine, which results in a concomitant influx of water into the cell, increases body water23,24,26–28 through a combination of increased extracellular fluid and intracellular fluid,23,24,26,29 and may have aided subjects by delaying the progression of dehydration.
Our purpose was to examine the influence of creatine supplementation on heat tolerance in dehydrated males during prolonged exercise interspersed with high-intensity bouts. This scenario is important because of the high incidence of creatine use among athletes who participate in demanding sports activities in which there is potential for thermal stress and because dehydration accentuates the thermal and cardiovascular stress of exercise in the heat30 and is recognized as an intrinsic risk factor for heat illness.31,32 We hypothesized that creatine supplementation would not alter thermoregulatory, cardiorespiratory, metabolic, or perceptual responses.
METHODS
Design Overview
A double-blind, randomized, placebo, crossover design was used. The study consisted of 2 duplicate trials (with the exception of the treatment), each lasting 10 days. A random sample of 6 subjects consumed 21.6 g of placebo (18 capsules) per day for 9 days, and 6 subjects consumed 21.6 g of CrM (18 capsules, Mega Creatine Fuel, Twinlab Laboratories, Inc, Ronkonkoma, NY) per day for 9 days. Subjects consumed 21.6 g·d−1 because this is equivalent to a typical recommended loading-phase dose (eg, 20 g·d−1 for 5 to 7 days). Subjects consumed half of the dose with breakfast and half with lunch. Subjects crossed over to the alternate treatment after a 48 ± 10 day washout period. On day 7 of each treatment, subjects reported to the laboratory at 7:00 am (12 hours postprandial) to perform an exercise heat-tolerance test (EHT).
We requested that subjects maintain their normal exercise regimens throughout the study, except on day 7 of each treatment, when the EHT was performed. Subjects were asked to maintain the same dietary habits during the study and to keep daily diet records during both trials. During the second trial, they were asked to replicate, as closely as possible, their diets as recorded during the first trial. Subjects were provided with detailed written and verbal instructions regarding recording of food and fluid intake before and during the study.
Subjects
Twelve non–heat-acclimated, physically active males (age = 22 ± 1 years, height = 180 ± 3 cm, mass = 78.8 ± 1.2 kg, body fat = 9 ± 1%, V̇o2peak = 50.9 ± 1 mL·kg−1·min−1) served as subjects. Possible participants were excluded if they had been exposed to high ambient temperature for at least 1 week within the previous 2 months before the study. Before testing, each subject reported, via a training history questionnaire, that he participated in a minimum of 8 hours of exercise training per week, the intensity of which was subjectively rated as moderate to intense, and that he maintained this level of activity during his involvement in the study. Additionally, screening information was obtained via a medical history questionnaire, to ensure that subjects had no history of (1) chronic health problems, (2) exertional heat illness, (3) cardiovascular, metabolic, or respiratory disease, or (4) creatine use during the past year. Subjects received verbal and written instructions of the testing procedures and the benefits and risks of the study and signed an informed consent document that had been approved by the Institutional Review Board for Studies Involving Human Subjects, which also approved the study.
Preliminary Tests
Before the first trial, subjects' peak oxygen uptake (V̇o2peak) and body composition were assessed. Skinfold thickness was measured on the right side of the body at the chest, abdomen, and thigh using calipers (Holtain Ltd, Crymych, UK). We used the equation of Jackson and Pollock33 to determine body density and the Siri34 equation to calculate percentage of body fat. The V̇o2peak was assessed with a progressive treadmill protocol graded by acceleration and conducted in a 27°C environment. Treadmill speed started at 9.66 km·h−1 and was increased by 1.61 km·h−1 every 2 minutes until the subjects reached volitional fatigue. Oxygen consumption (V̇o2) was measured throughout the test using online, open-circuit spirometry (model CPX-D; Medical Graphics, Inc, St. Paul, MN) calibrated according to the manufacturer's guidelines before each test.
Testing Protocol, Day 7
On day 7, the subjects entered the environmental chamber (temperature = 33.5 ± 0.5°C, relative humidity = 41 ± 12%; model 2000; Minus-Eleven, Inc, Malden, MA) and remained there until completion of the EHT. Subjects consumed half of their daily treatment dose (CrM or placebo) and then positioned a rectal thermistor. An 18-gauge Teflon (Du Pont, Wilmington, DE) catheter was placed in a superficial forearm vein, and the subject then stood for 20 minutes to allow equilibration of body fluids. While standing, subjects completed an Environmental Symptoms Questionnaire (ESQ), after which blood was drawn to allow analyses of hematocrit, hemoglobin, plasma osmolality, plasma lactate, total plasma protein, plasma glucose, plasma sodium, and plasma potassium. Subjects then provided a urine sample (for assessment of urine osmolality, urine specific gravity, urine color, and urine volume) and were weighed in shorts on an electronic scale to the nearest 50 g (model 700M; SR Instruments, Tonawanda, NY).
Subjects then performed 120 minutes of submaximal exercise by alternating 30 minutes of walking (6.6 ± 0.32 km·h−1) on a treadmill with 30 minutes of cycling (model 818E; Monark, Stockholm, Sweden). During cycling, power output was modified to elicit a heart rate response similar to walking. The goal was to dehydrate the subjects by 2.0% of body mass.35
After exercise, subjects dried their skin surfaces and were weighed as before. They then sat quietly in the chamber and consumed a standardized meal that consisted of a commercially available nutrition bar (Gatorade energy bar; Gatorade, Chicago, IL) containing 261 calories, 46 g carbohydrate, 8 g protein, and 5 g fat; 200 mL of water; and their midday doses of CrM or placebo capsules. Thirty minutes after completing the meal, subjects provided a urine sample and were weighed.
An hour after completing the dehydration protocol, subjects stood for 30 minutes before blood and urine samples were collected and analyzed as described above. Additionally, heart rate, blood pressure, and rectal and skin temperature (at the calf, thigh, chest, and triceps) were recorded. Mean weighted skin temperature was later calculated using the skin temperature values in accordance with the 4-site method of Ramanathan.36 Subjects then performed 80 minutes of exercise that consisted of four 20-minute sequences of standing for 4 minutes, alternating a 3-minute walk (6.6 ± 0.3 km·h−1, 37.0 ± 5.8% V̇o2peak) and a 1-minute high-intensity run (19.0 ± 0.6 km·h−1, 114.9 ± 5.3 % V̇o2peak) 3 times, and walking (6.6 ± 0.3 km·h−1, 37.0 ± 5.8 V̇o2peak) for 4 minutes. During the 4-minute rest, a blood sample was taken, and ratings of perceived exertion (of lower-body [leg] exertion, central [respiratory] exertion, and overall [whole body] exertion); thirst; thermal sensation; heart rate; blood pressure; and rectal and skin temperatures were recorded. At minutes 17, 37, 57, and 77, V̇o2, respiratory exchange ratio, minute ventilation, and respiratory rate were measured continuously for 3 minutes using online, open-circuit spirometry. Figure 1 illustrates the day 7 protocol.
Figure 1. Schematic representation of the day 7 protocol. S indicates standing; BM, body mass; ESQ, Environmental Symptoms Questionnaire; EHT, exercise heat-tolerance test; and RPE, rating of perceived exertion.
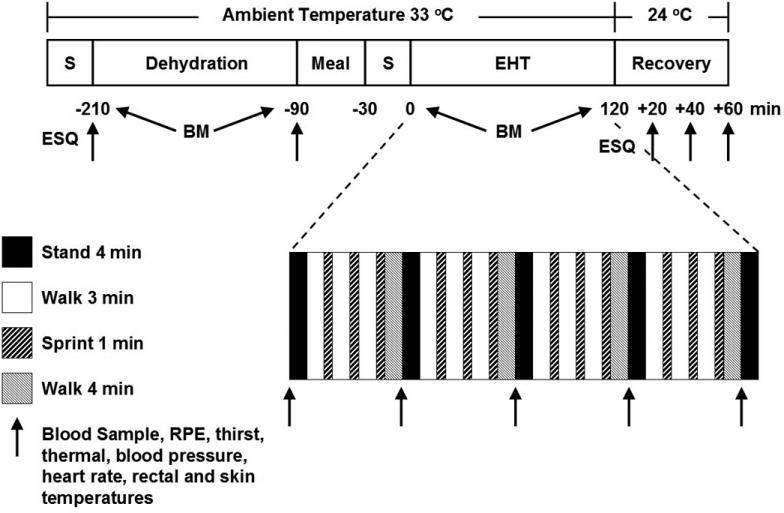
Air flow (2.3 m·s−1) was generated by a fan directed at the subject during the EHT. All timekeeping devices were hidden from the subject's view. The exercise session was terminated if subjects stopped because of volitional fatigue, had rectal temperature ≥40°C, had heart rate ≥185 beats·min−1 for 5 consecutive minutes, exhibited signs and symptoms of heat illness, or could not continue running at the predetermined speed. One subject became exhausted and was stopped from exercising at 60 minutes during his first EHT; consequently, he was stopped at 60 minutes during his second EHT. After exercise, subjects completed an ESQ and then walked from the environmental chamber into a cooler environment (24°C) and consumed 1 L of water within 10 minutes. They remained standing for 1 hour, during which time blood was drawn and lower body, central, and overall ratings of perceived exertion; thirst; thermal sensation; skin and rectal temperatures (for calculation of mean weighted skin temperature); heart rate; and blood pressure were recorded every 20 minutes.
Instrumentation and Measurement Techniques
Rectal temperature was measured using a rectal thermistor (model 401; Yellow Springs Instruments, Yellow Springs, OH) inserted 10 cm past the anal sphincter. Skin temperature was measured using an infrared tympanic temperature scanner (model HTTS-3000; Exergen Corp, Newton, MA).37 A Physiological Strain Index was calculated to evaluate heat stress using rectal temperature and heart rate according to the formulas of Moran et al.38
Heart rate was measured via telemetry (Polar Vantage; Polar Electro, Port Washington, NY). Blood pressure was obtained via an aneroid sphygmomanometer and stethoscope over the brachial artery. Mean arterial pressure was calculated as diastolic blood pressure + 1/3 (systolic blood pressure − diastolic blood pressure).
Perceived thirst was identified using a 9-point thirst scale with verbal anchors ranging from 1 (not thirsty at all) to 9 (very, very thirsty).39 Thermal sensation was identified using a 17-point scale ranging from 0 (unbearably cold) to 8 (unbearably hot) in 0.5-point intervals.40 Ratings of perceived exertion were obtained using the 15-point scale.41
Blood samples were acquired from a superficial forearm vein with a flexible 18-gauge Teflon catheter and an attached Luer adapter. The catheter port and male Luer adapter were kept patent with 1.5 mL of a heparin flush (10%) solution after each sample collection. Hematocrit was determined in triplicate from whole blood by the microcapillary technique. Hemoglobin was measured in triplicate by the cyanmethemoglobin method (kit 525; Sigma Chemical, Inc, St. Louis, MO) using a spectrophotometer (Spectronic 88; Bausch & Lomb, Rochester, NY). Percentage change in plasma volume was calculated using the method of Dill and Costill.42 Plasma and urine osmolality were measured in duplicate with a freezing-point depression osmometer (model 3DII; Advanced Instruments, Inc, Needham Heights, MA). Sodium and potassium levels in plasma and urine were measured in duplicate via ion-sensitive electrodes (model 984-S; AVL Scientific Corp, Roswell, GA). Plasma glucose and lactate were determined in triplicate using enzymatic techniques (model 2003, Glucose/ Lactate Analyzer; Yellow Springs Instruments, Inc, Yellow Springs, OH). Urine specific gravity and total plasma protein were measured by refractometry (model A300CL; Spartan, Tokyo, Japan). Urine color was determined according to the technique described by Armstrong et al.43 The urine color chart has been published in the National Athletic Trainers' Association position statement on fluid replacement for athletes.35 At 7:00 am on days 8–10 of each treatment, subjects reported to the laboratory for an assessment of their health and hydration status.
Statistical Analysis
Data are reported as mean ± standard deviation. To evaluate the effects of CrM on the experimental variables, 2-way within-subjects analyses of variance (ANOVAs) were used to evaluate for significant interaction and main effect differences during dehydration, the EHT, and recovery from the EHT. The independent variables were treatment, with 2 levels (CrM and placebo), and time (for which the number of levels differed by variable and period, ie, dehydration, EHT, and recovery). The alpha level for statistical significance was set at P < 0.05. All significant time effects and interactions were analyzed using paired t tests with sequential Bonferroni corrections. All statistics were computed with the Statistical Package for Social Sciences (version 11.0 for Windows; SPSS Inc, Chicago, IL).
RESULTS
Body Mass
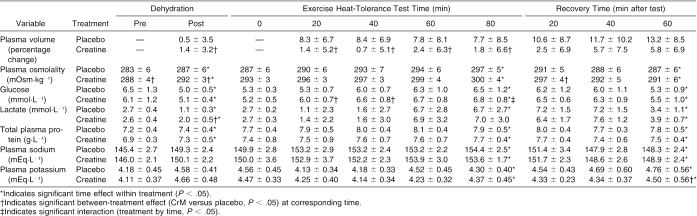
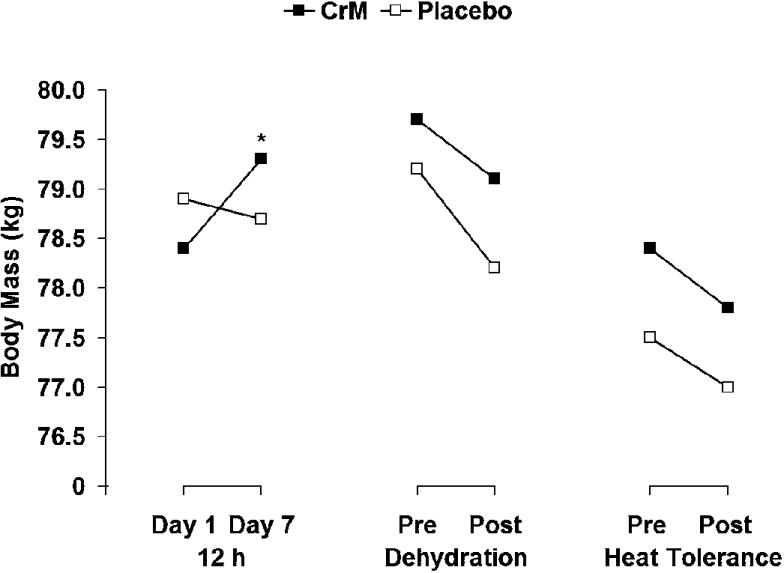
A significant interaction (F5,50 = 6.435, P = <0.001, η2= 0.392) but no significant main effect for treatment (F1,10 = 2.973, P = 0.115, η2= 0.229) was noted. Follow-up analysis indicated a significant interaction in the change in body mass from day 1 to day 7 (t11 = −2.898, P = 0.015), as illustrated in Figure 2. During dehydration, body mass was reduced by −1.9 ± 0.5% and −2.0 ± 0.5% during placebo and CrM, respectively. The cumulative loss in body mass (measured from before dehydration to after EHT) was −4.09 ± 0.53% during CrM and −4.38 ± 0.58% during placebo. Total sweat losses were similar during the placebo and creatine conditions, as indicated by similar decreases in body mass during dehydration exercise (placebo = 1.5 ± 0.4 kg, CrM = 1.6 ± 0.4 kg, t10 = 0.616, P = .552) and the EHT (placebo = 0.8 ± 0.3 kg, CrM = 1.0 ± 0.4 kg, t11 = 1.423, P = .183).
Figure 2. Changes in body mass as a result of supplementation, dehydration, and the exercise heat-tolerance test. *Significant interaction, CrM indicates creatine monohydrate. P < .05.
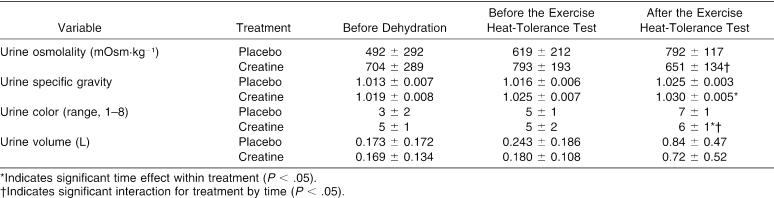
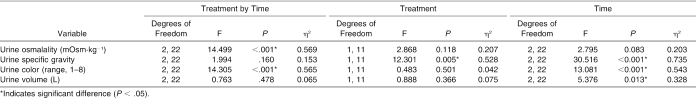
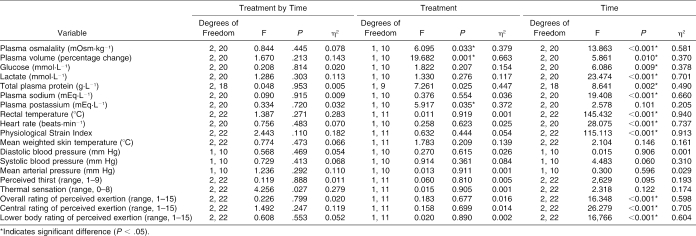
Urine
Urine osmolality, urine specific gravity, urine color, and urine volume values are presented in Table 1 and the ANOVA summary statistics in Table 2. Significant interactions were seen in urine osmolality and urine color, with a treatment difference in urine specific gravity. Follow-up analysis indicated that urine color increased during placebo but remained similar during CrM from before dehydration to before the EHT (t11 = −3.811, P = 0.003) and from before to after the EHT (t11 = −4.109, P = 0.002). Also, urine color was higher before dehydration during placebo (t11 = −3.246, P = 0.008). Urine osmolality increased during placebo but remained similar during CrM from before dehydration to before the EHT (t11 = −4.310, P = 0.001) and increased during placebo but decreased during CrM from before to after the EHT (t11 = −4.109, P = 0.002). Urine specific gravity was significantly higher during CrM before dehydration (t11 = −2.500, P = 0.030), before the EHT (t11 = −3.578, P = 0.004), and after the EHT (t11 = −3.180, P = 0.009).
Table 1. Urinary Responses Before Dehydration and Before and After the Exercise Heat-Tolerance Test.
Table 2. Analysis of Variance Summary Statistics for Urine Osmolality, Specific Gravity, Volume, and Color.
Environmental Symptoms Questionnaire Scores
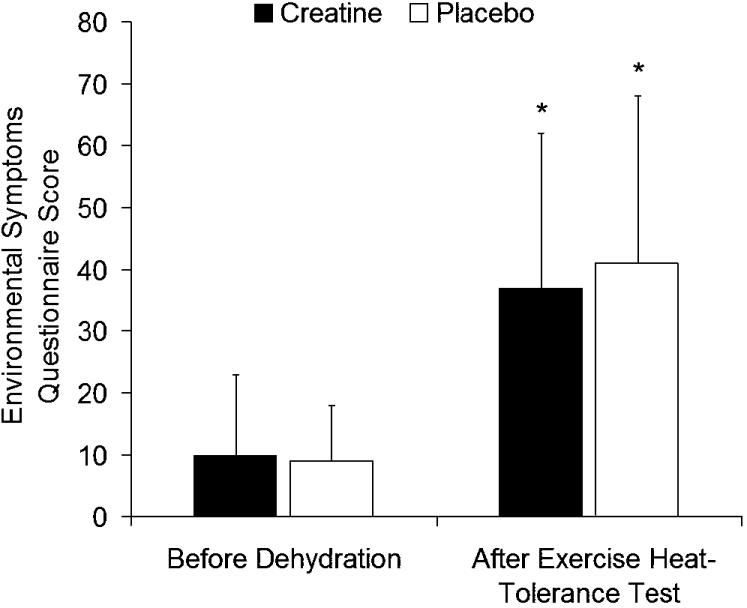
No interaction (F1,10 = 0.623, P = 0.448, η2 = 0.059) or treatment effect (F1,10 = 0.402, P = 0.540, η2 = 0.039) was demonstrated, but scores were significantly increased after exercise (F1,10 = 42.639, P < 0.001, η2 = 0.810) (Figure 3).
Figure 3. Environmental Symptoms Questionnaire scores recorded before dehydration and after the exercise heat-tolerance test. No interaction or treatment differences were noted. *Scores were increased after the exercise heat-tolerance test.
Dehydration Exercise
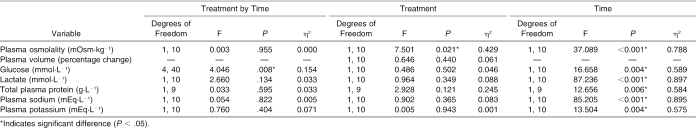
The ANOVA summary statistics for all variables measured during dehydration are presented in Table 3. Only a significant interaction for plasma glucose and a treatment main effect for plasma osmolality were identified (Table 3). Follow-up analysis indicated that plasma glucose decreased more during CrM than during placebo (t9 = 3.826, P = 0.004), and plasma osmolality was significantly higher during CrM before (t10 = −2.495, P = 0.032) and after dehydration (t10 = −2.921, P = 0.015). No other significant interactions or treatment effects for the variables measured before and after dehydration were seen.
Table 3. Analysis of Variance Summary Statistics for Variables Measured During Dehydration.
Exercise Heat-Tolerance Test
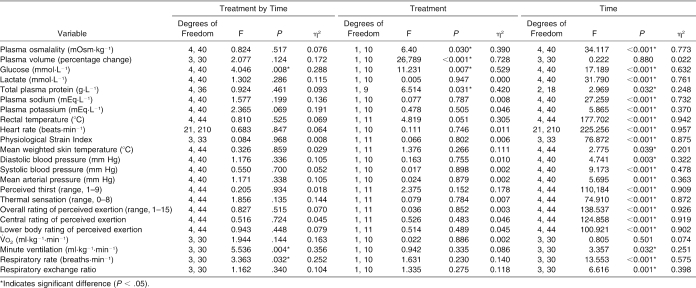
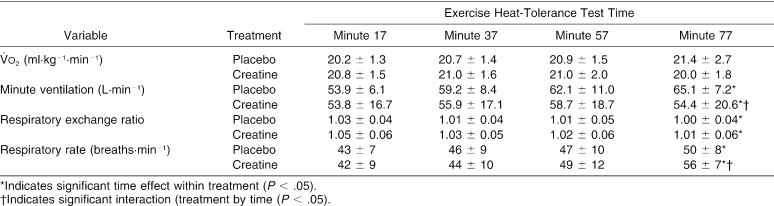
The ANOVA summary statistics for all variables measured during the EHT are presented in Table 4. Significant interactions were identified for plasma glucose, minute ventilation, and respiratory rate. Follow-up analysis indicated that plasma glucose increased significantly more with CrM during the first 20 minutes of exercise than with placebo (t10 = 4.348, P = 0.001), minute ventilation increased significantly more with placebo from minute 17 to minute 77 than with CrM (t10 = −3.286, P = 0.008), and respiratory rate increased significantly more with CrM from minute 37 to minute 77 than with placebo (t10 = 3.355, P = 0.008).
Table 4. Analysis of Variance Summary Statistics for the Variables Measured During the Exercise Heat-Tolerance Test.
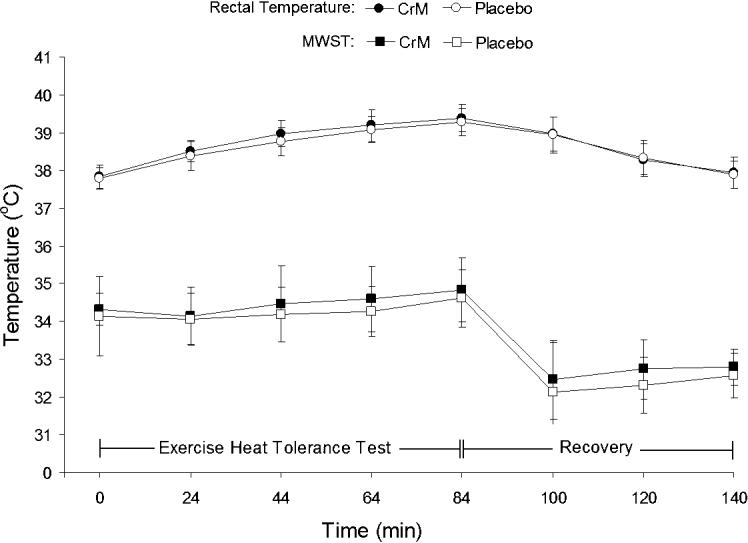
Significant treatment main effects were identified for plasma glucose, percentage change in plasma volume, plasma osmolality, and total plasma protein. Follow-up analysis showed that plasma glucose was significantly higher during CrM at minutes 20 (t10 = −7.578, P = <0.001) and 40 (t10 = −4.485, P = 0.001) than with placebo. The percentage change in plasma volume was significantly greater with placebo than with CrM at minutes 20 (t10 = 5.828, P = <0.001), 40 (t10 = 7.127, P = <0.001), 60 (t10 = 3.260, P = 0.009) and 80 (t10 = 3.753, P = 0.004). No differences were demonstrated with follow-up analysis of the treatment effect in plasma osmolality and total plasma protein. Figure 4 illustrates the similar heart rate responses, and Figure 5 illustrates the similar rectal temperature and mean weighted skin temperature responses during CrM and placebo.
Figure 4. Heart rate responses during the exercise heat-tolerance test and recovery. No interaction or treatment differences were noted. CrM indicates creatine monohydrate.
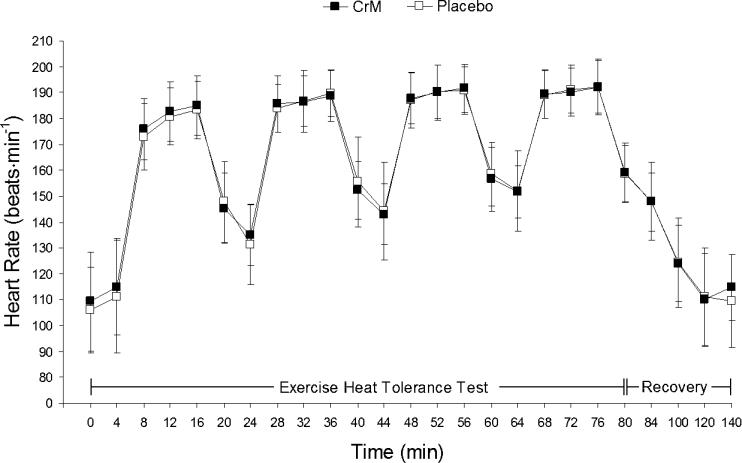
Figure 5. Rectal temperature and mean weighted skin temperature (MWST) responses during the exercise heat-tolerance test and recovery. No interaction or treatment differences were noted. CrM indicates creatine monohydrate.
Recovery
The ANOVA summary statistics for the variables measured during recovery are presented in Table 5. Significant treatment main effects were identified for plasma osmolality, percentage change in plasma volume, and plasma potassium. Follow-up analysis indicated that plasma osmolality was significantly greater with CrM 20 minutes into recovery compared with placebo (t10 = −3.321, P = 0.008), and percentage change in plasma volume was significantly greater with placebo at 20 (t10 = 5.008, P = 0.001), 40 (t10 = 3.184, P = 0.010), and 60 (t10 = 4.356, P = 0.001) minutes into recovery compared with CrM. No differences were indicated by follow-up analysis to the treatment effect in plasma potassium.
Table 5. Analysis of Variance Summary Statistics for the Variables Measured During Recovery.
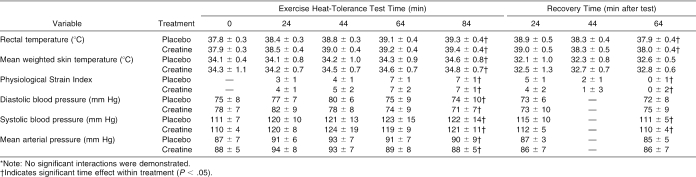
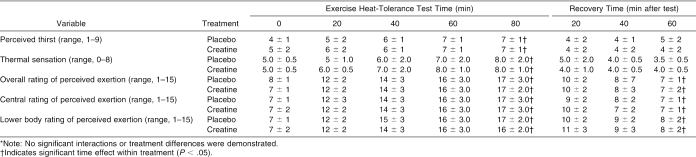
Thermoregulatory and blood pressure responses before and after dehydration and during the EHT and recovery are presented in Table 6. Thermoregulatory and blood pressure responses during the EHT and recovery are presented in Table 7. Perceptual responses during the EHT and recovery are presented in Table 8. Cardiopulmonary responses during the EHT are displayed in Table 9.
Table 6. Metabolic and Hematologic Responses Before and After Dehydration and During the Exercise Heat-Tolerance Test and Recovery.
Table 7. Thermoregulatory and Blood Pressure Responses During the Exercise Heat-Tolerance Test and Recovery*.
Table 8. Perceptual Responses During the Exercise Heat-Tolerance Test and Recovery*.
Table 9. Cardiopulmonary Responses During the Exercise Heat-Tolerance Test.
DISCUSSION
Concern has been expressed about possible alterations in fluid balance and impairment of thermoregulation in athletes acutely loading with creatine in settings with the potential for thermal stress.20 To examine this, we compared thermoregulatory (rectal temperature, mean weighted skin temperature, sweat loss), cardiopulmonary (V̇o2, minute ventilation, respiratory exchange ratio, respiratory rate, heart rate, blood pressure), metabolic (plasma glucose, lactate), and perceptual responses (thirst, thermal sensation, ratings of perceived exertion) in dehydrated men during 80 minutes of exercise (a typical length of time of a practice session or game in numerous sports) interspersed with high-intensity exercise bouts after a week of either CrM or placebo supplementation.
We found that CrM was associated with better maintenance of plasma volume and slightly higher (but normal) plasma glucose during exercise. Additionally, we found that urine osmolality and urine color were greater before exercise and that urine specific gravity was higher before and after exercise with CrM, indicative of increased creatine and creatinine excretion during CrM. These urine data are important because if urine specific gravity and/or urine color (2 common hydration indices) are used to assess the hydration state of athletes supplementing with CrM, then these athletes may appear more dehydrated or hypohydrated than they actually are.
No difference was noted in the ESQ scores before and after exercise between CrM and placebo, suggesting that short-term creatine use was not associated with any symptomatic side effects. Of particular interest, the incidence of cramping was not increased with CrM, probably because there were no abnormalities in plasma sodium and potassium, no difference in dehydration level incurred during exercise (CrM versus placebo), and no metabolic abnormalities, all of which have been presented as hypotheses to explain muscle cramping.44 Our data, therefore, show that hydration state and thermoregulation were not adversely affected by short-term CrM supplementation in these men exercising in the heat, nor did CrM increase the incidence of symptoms of heat illness compared with placebo.
We found that 1 week of CrM supplementation increased body mass an average of 0.88 kg versus no change with placebo. This is consistent with previous findings (+0.7 to 2.0 kg),22,24,27,45,46 although significant increases are not always observed after a 7-day loading phase.27 The body mass gain likely involved water retention, which occurs as a consequence of the osmotic load of CrM, as evidenced by the significantly higher plasma osmolality with CrM versus placebo at rest before dehydration (289 versus 283 mOsm·kg−1, P < 0.001) and during the EHT (296 versus 291 mOsm·kg−1, P = 0.028). Small increases in contractile protein synthesis have been observed after CrM supplementation,47 but the contribution to increased body mass is minimal compared with water retention, and the subjects in this study were not performing high-intensity activity. Previous researchers27 have shown that during creatine supplementation, retained fluid is distributed normally between the fluid compartments. In our study, then, it is likely that approximately two thirds of the body mass gain resulted from water retained in the intracellular fluid space and that the remaining third of the gain was because of fluid retained in the extracellular fluid space. Consequently, plasma volume may have increased slightly (∼50 mL) with CrM.48
Dehydration resulted in similar losses of body mass and plasma volume for CrM and placebo. During the initial 20 minutes of the EHT, however, plasma volume was significantly reduced with placebo but not with CrM; both values decreased similarly thereafter until the end of exercise. This result contrasts with the findings of Volek et al,22 who observed no difference in percentage change of plasma volume during 35 minutes of intense cycling in the heat. Our data suggest that creatine use may limit the movement of fluid out of the plasma and into the intracellular fluid space at the onset of exercise in the heat, when subjects are dehydrated. However, the maintenance of plasma volume and the increase in total body water were not associated with a change in sweat loss or with decreases in rectal temperature, heart rate, blood pressure, or perceptual responses (thirst, thermal sensation, and ratings of perceived exertion) and, therefore, CrM did not provide a measurable hydration or thermoregulatory advantage. It is unclear why plasma volume was maintained with CrM, but extracellular volume may have been maintained as a consequence of the osmotic property of creatine and increased plasma osmolality.
In agreement with previous findings22,24 and as indicated by similar body mass losses for CrM and placebo, the overall level of dehydration attained by the end of exercise was not affected by CrM. Consequently, athletes should not be concerned that creatine supplementation alone may contribute to whole-body dehydration while exercising for prolonged periods under significant thermal stress.
In this study, CrM supplementation had no effect on V̇o2, plasma lactate, or respiratory exchange ratio, which is in agreement with previous research examining creatine use during exercise in the heat.24 Contrasting results for V̇o2 during submaximal exercise in a temperate environment have been observed; however, because V̇o2 either was unaltered49 or was attenuated (along with lactate accumulation),50 neither necessitate concern about CrM use. Our data support the suggestion of Kilduff et al24 that muscle bioenergetics are unaffected by creatine during exercise in the heat, and ours are the first data to show this in dehydrated men.
Currently, no evidence exists to support an independent and contrasting effect of CrM on minute ventilation and respiratory rate. Consequently, as there were no interaction or treatment differences in V̇o2, plasma lactate, or respiratory exchange ratio, the contrasting interactions found for minute ventilation (increasing more in placebo) and respiratory rate (increasing more in CrM) are difficult to explain. Both effect-size statistics (η2) for these interactions were weak, however.
The elevation of plasma glucose with CrM is also difficult to explain. However, evidence suggests that creatine supplementation disrupts glucose homeostasis. For example, Rooney et al51 showed elevated blood glucose in subjects after they ingested a 75-g glucose load following creatine supplemention for 42 days. The changes in plasma glucose were independent of changes in plasma insulin, but a mechanism to explain the disruption in glucose homeostasis was not established. It is possible that a similar disruption in glucose homeostasis resulted from the 46 g of carbohydrate contained in the meal eaten 90 minutes before the EHT. In neither study, however, was plasma glucose ever abnormally high.
We found no effect of CrM on exercise heart rate and blood pressure responses, and to our knowledge, these are the first data to show these results with the added component of moderate dehydration. Previous research shows contrasting findings: creatine supplementation during exercise in the heat either had no effect on heart rate and blood pressure22 or attenuated heart rate.24 Methodologic differences (duration and intensity) likely explain these contrasting results, but again, cause for concern about short-term CrM supplementation in this population was not supported.
Body temperature (rectal temperature and mean weighted skin temperature), thermal sensation, and the Physiological Strain Index (a criterion of heat strain)38 were not altered by CrM supplementation. Previous authors who have examined creatine use and exercise in the heat have shown that body temperature responses are similar to placebo22 or that creatine attenuated the rise in body temperature versus placebo.23–25 Our data extend these findings and are the first to indicate that creatine does not significantly affect temperature regulation and cardiovascular responses in trained, dehydrated men performing intense exercise under high thermal stress.
Overall (ie, whole body exertion), central (ie, exertion associated with breathing) and lower body (ie, leg exertion) ratings of perceived exertion were not affected by short-term CrM supplementation. Kilduff et al,24 however, found that creatine supplementation significantly reduced ratings of perceived leg fatigue during exercise and increased the ease of exercise after supplementation. In contrast to our study, Kilduff et al also found that creatine significantly decreased heart rate, rectal temperature, and sweat rate to provide a mechanism for reduced heat strain and dehydration during exercise.
Acute urine volume was unaltered by CrM supplementation but urine osmolality was higher during CrM supplementation. Previous investigators have reported increased22 and decreased3 24-hour urine volumes after a week of creatine loading. The CrM altered urine color, urine specific gravity, and urine osmolality. These changes were likely because of increases in the urinary content of creatinine52 and creatine53 resulting from creatine supplementation.
CONCLUSIONS
Our data add to the growing number of studies showing that short-term CrM supplementation does not adversely affect thermoregulatory, cardiorespiratory, metabolic, or perceptual responses in people exercising under thermal stress. Our study was the first to show this in dehydrated individuals. Furthermore, short-term CrM supplementation was not associated with increased incidence of negative side effects (ie, cramping or heat illnesses and injuries). Consequently, concern for trained men who supplement with creatine, at least in the short term, and exercise under thermal stress remains unfounded. The general recommendation for people not to use creatine while exercising under thermal stress should be reconsidered.
Acknowledgments
We thank the 12 dedicated subjects for their commitment to the study. Also, we gratefully acknowledge the technical assistance provided by Dr Jeff Volek, Micheal D'Alfonso, Katie Krog, and Alex Seen. This study was funded by a grant awarded by the National Athletic Trainers' Association Research & Education Foundation.
REFERENCES
- Febbraio MA, Flanagan TR, Snow RJ, Zhao S, Carey MF. Effect of creatine supplementation on intramuscular TCr, metabolism and performance during intermittent, supramaximal exercise in humans. Acta Physiol Scand. 1995;155:387–395. doi: 10.1111/j.1748-1716.1995.tb09988.x. [DOI] [PubMed] [Google Scholar]
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol. 1996;271:E31–E37. doi: 10.1152/ajpendo.1996.271.1.E31. (1 pt 1) [DOI] [PubMed] [Google Scholar]
- Maughan RJ. Creatine supplementation and exercise performance. Int J Sport Nutr. 1995;5:94–101. doi: 10.1123/ijsn.5.2.94. [DOI] [PubMed] [Google Scholar]
- Rawson ES, Volek JS. Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J Strength Cond Res. 2003;17:822–831. doi: 10.1519/1533-4287(2003)017<0822:eocsar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bogdanis GC, Nevill ME, Lakomy HK, Boobis LH. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol Scand. 1998;163:261–272. doi: 10.1046/j.1365-201x.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Chwalbinska-Moneta J. Effect of creatine supplementation on aerobic performance and anaerobic capacity in elite rowers in the course of endurance training. Int J Sport Nutr Exerc Metab. 2003;13:173–183. doi: 10.1123/ijsnem.13.2.173. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y, Hamaoka T, Katsumura T. Creatine supplementation enhances anaerobic ATP synthesis during a single 10 sec maximal handgrip exercise. Mol Cell Biochem. 2003;244:105–112. et al. [PubMed] [Google Scholar]
- Kreider RB. Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem. 2003;244:89–94. [PubMed] [Google Scholar]
- Smith J, Dahm DL. Creatine use among a select population of high school athletes. Mayo Clin Proc. 2000;75:1257–1263. doi: 10.4065/75.12.1257. [DOI] [PubMed] [Google Scholar]
- McGuine TA, Sullivan JC, Bernhardt DT. Creatine supplementation in high school football players. Clin J Sport Med. 2001;11:247–253. doi: 10.1097/00042752-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Metzl JD, Small E, Levine SR, Gershel JC. Creatine use among young athletes. Pediatrics. 2001;108:421–425. doi: 10.1542/peds.108.2.421. [DOI] [PubMed] [Google Scholar]
- LaBotz M, Smith BW. Creatine supplement use in an NCAA Division I athletic program. Clin J Sport Med. 1999;9:167–169. doi: 10.1097/00042752-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Greenwood M, Farris J, Kreider R, Greenwood L, Byars A. Creatine supplementation patterns and perceived effects in select Division I collegiate athletes. Clin J Sport Med. 2000;10:191–194. doi: 10.1097/00042752-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Trulock S. A survey of creatine use and related trends in injury pattens in professional football. Pro Football Athl Trainer. 1999;17:4–5. [Google Scholar]
- Sheppard HL, Raichada SM, Kouri KM, Stenson-Bar-Maor L, Branch JD. Use of creatine and other supplements by members of civilian and military health clubs: a cross-sectional survey. Int J Sport Nutr Exerc Metab. 2000;10:245–259. doi: 10.1123/ijsnem.10.3.245. [DOI] [PubMed] [Google Scholar]
- Bailes JE, Cantu RC, Day AL. The neurosurgeon in sport: awareness of the risks of heatstroke and dietary supplements. Neurosurgery. 2002;51:283–288. [PubMed] [Google Scholar]
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Terjung RL, Clarkson P, Eichner ER. American College of Sports Medicine roundtable: the physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. et al. [DOI] [PubMed] [Google Scholar]
- Greenwood M, Kreider RB, Melton C. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Mol Cell Biochem. 2003;244:83–88. et al. [PubMed] [Google Scholar]
- Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gomez AL, Kraemer WJ. Physiological responses to short-term exercise in the heat after creatine loading. Med Sci Sports Exerc. 2001;33:1101–1108. doi: 10.1097/00005768-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Pitsiladis YP, Georgiades E, Minnion RH, Mitchell M, Kingsmore D, Kilduff LP. Effects of creatine supplementation on exercise performance in the heat in endurance-trained humans [abstract] Med Sci Sports Exerc. 2003;35:S32. doi: 10.1123/ijsnem.14.4.443. [DOI] [PubMed] [Google Scholar]
- Kilduff LP, Georgiades E, James N. The effects of creatine supplementation on cardiovascular, metabolic, and thermoregulatory responses during exercise in the heat in endurance-trained humans. Intl J Sport Nutr Exerc Metab. 2004;14:443–460. doi: 10.1123/ijsnem.14.4.443. et al. [DOI] [PubMed] [Google Scholar]
- Kern M, Podewils LJ, Vukovich M, Buono J. Physiological response to exercise in the heat following creatine supplementation. JEPonline. 2001;4:18–27. Available at: www.css.edu/asep. [Google Scholar]
- Ziegenfuss TN, Lowery LM, Lemon PW. Acute fluid volume changes in men during three days of creatine supplementation. JEPonline. 1998;1:3. Available at: www.css.edu/asep. [Google Scholar]
- Powers ME, Arnold BL, Weltman AL. Creatine supplementation increases total body water without altering fluid distribution. J Athl Train. 2003;38:44–50. et al. [PMC free article] [PubMed] [Google Scholar]
- Kutz MR, Gunter MJ. Creatine monohydrate supplementation on body weight and percent body fat. J Strength Cond Res. 2003;17:817–821. doi: 10.1519/1533-4287(2003)017<0817:cmsobw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brilla LR, Giroux MS, Taylor A, Knutzen KM. Magnesium-creatine supplementation effects on body water. Metabolism. 2003;52:1136–1140. doi: 10.1016/s0026-0495(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J. Separate and combined influences of dehydration and hyperthermia on cardiovascular responses to exercise. Int J Sports Med. 1998;19:S111–S114. doi: 10.1055/s-2007-971972. (suppl 2) [DOI] [PubMed] [Google Scholar]
- Binkley HM, Beckett J, Casa DJ, Kleiner DM, Plummer PE. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37:329–343. [PMC free article] [PubMed] [Google Scholar]
- Casa DJ, Almquist J, Anderson S. Inter-Association Task Force on Exertional Heat Illnesses consensus statement. et al. NATA News. June 2003; 24–29.
- Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- Siri W. Body Composition From Fluid Spaces and Density: Analysis of Methods. Washington, DC: National Academy of Science; 1961. [PubMed]
- Casa DJ, Armstrong LE, Hillman SK. National Athletic Trainers' Association position statement: fluid replacement for athletes. J Athl Train. 2000;35:212–224. et al. [PMC free article] [PubMed] [Google Scholar]
- Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Maresh CM, Gabaree CV. Thermal and circulatory responses during exercise: effects of hypohydration, dehydration, and water intake. J Appl Physiol. 1997;82:2028–2035. doi: 10.1152/jappl.1997.82.6.2028. et al. [DOI] [PubMed] [Google Scholar]
- Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:R129–R134. doi: 10.1152/ajpregu.1998.275.1.R129. (1 Pt 2) [DOI] [PubMed] [Google Scholar]
- Engell DB, Maller O, Sawka MN, Francesconi RN, Drolet L, Young AJ. Thirst and fluid intake following graded hypohydration levels in humans. Physiol Behav. 1987;40:229–236. doi: 10.1016/0031-9384(87)90212-5. [DOI] [PubMed] [Google Scholar]
- Toner MM, Drolet LL, Pandolf KB. Perceptual and physiological responses during exercise in cool and cold water. Percept Mot Skills. 1986;62:211–220. doi: 10.2466/pms.1986.62.1.211. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Soto JA, Hacker FT, Jr, Casa DJ, Kavouras SA, Maresh CM. Urinary indices during dehydration, exercise, and rehydration. Int J Sport Nutr. 1998;8:345–355. doi: 10.1123/ijsn.8.4.345. [DOI] [PubMed] [Google Scholar]
- Schwellnus MP, Derman EW, Noakes TD. Aetiology of skeletal muscle ‘cramps’ during exercise: a novel hypothesis. J Sports Sci. 1997;15:277–285. doi: 10.1080/026404197367281. [DOI] [PubMed] [Google Scholar]
- Volek JS, Kraemer WJ, Bush JA. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J Am Diet Assoc. 1997;97:765–770. doi: 10.1016/S0002-8223(97)00189-2. et al. [DOI] [PubMed] [Google Scholar]
- Mujika I, Chatard JC, Lacoste L, Barale F, Geyssant A. Creatine supplementation does not improve sprint performance in competitive swimmers. Med Sci Sports Exerc. 1996;28:1435–1441. doi: 10.1097/00005768-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Balsom PD, Ekblom B, Soderlund K, Sjodin B, Hultman E. Creatine supplementation and dynamic high-intensity intermittent exercise. Scand J Med Sci Sports. 1993;3:143–149. [Google Scholar]
- Koeppen B, Stanton B. Renal Physiology. 3rd ed. St. Louis, MO: CV Mosby Co Year Book; 2000.
- Stroud MA, Holliman D, Bell D, Green AL, Macdonald IA, Greenhaff PL. Effect of oral creatine supplementation on respiratory gas exchange and blood lactate accumulation during steady-state incremental treadmill exercise and recovery in man. Clin Sci (Lond) 1994;87:707–710. doi: 10.1042/cs0870707. [DOI] [PubMed] [Google Scholar]
- Jones AM, Carter H, Pringle JS, Campbell IT. Effect of creatine supplementation on oxygen uptake kinetics during submaximal cycle exercise. J Appl Physiol. 2002;92:2571–2577. doi: 10.1152/japplphysiol.01065.2001. [DOI] [PubMed] [Google Scholar]
- Rooney KB, Bryson JM, Digney AL, Rae CD, Thompson CH. Creatine supplementation affects glucose homeostasis but not insulin secretion in humans. Ann Nutr Metab. 2003;47:11–15. doi: 10.1159/000068908. [DOI] [PubMed] [Google Scholar]
- Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR. Effect of short-term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol. 1997;76:566–567. doi: 10.1007/s004210050291. [DOI] [PubMed] [Google Scholar]
- Harris RC, Almada AL, Harris DB, Dunnett M, Hespel P. The creatine content of Creatine Serum and the change in the plasma concentration with ingestion of a single dose. J Sports Sci. 2004;22:851–857. doi: 10.1080/02640410310001658739. [DOI] [PubMed] [Google Scholar]