Abstract
Context: A dehydrated individual who performs eccentric exercise may exacerbate skeletal muscle damage, leading to structural, contractile, and enzymatic protein denaturation, in addition to the myofiber and connective damage resulting from the eccentric muscle tension.
Objective: To identify the effects of dehydration on 5 physiologic characteristics of delayed-onset muscle soreness (DOMS) in normothermic men after an eccentric exercise perturbation.
Design: Randomized group test-retest design.
Setting: Laboratory.
Patients or Other Participants: Ten healthy male volunteers randomly assigned to either a euhydration (age = 26.2 ± 4.9 years, height = 174.1 ± 6.0 cm, mass = 86.5 ± 15.3 kg) or dehydration (age = 25.8 ± 2.2 years, height = 177.2 ± 3.1 cm, mass = 84.4 ± 3.8 kg) group.
Intervention(s): Subjects performed treadmill walking for 45 minutes in either a thermoneutral (euhydration) or a hot, humid (dehydration) environment. After a rest period to allow for return to the normothermic condition, DOMS was induced with a 45-minute downhill run.
Main Outcome Measures: We assessed 5 physiologic characteristics of DOMS before and at intervals after the eccentric exercise. The characteristics were perceived pain of the bilateral quadriceps and overall body, bilateral punctate tenderness of the superficial quadriceps muscles, bilateral knee-flexion passive range of motion, bilateral thigh circumference, and bilateral isometric quadriceps muscle strength. Thermoregulatory and cardiovascular measures were obtained to monitor participants' heat load during exercise.
Results: The experimental protocol produced a 0.9% increase in body mass of the euhydration group and a significant 2.7% decrease in body mass of the dehydration group. The downhill-running exercise perturbation induced DOMS in both the euhydrated and dehydrated participants, based on increased bilateral quadriceps and overall body perceived pain and punctate tenderness of the bilateral vastus medialis muscle. The signs and symptoms of DOMS after an eccentric exercise perturbation were not exacerbated by moderate dehydration of 2.7% body mass after rest and return to the normothermic condition.
Conclusions: Significantly dehydrated participants who rested and returned to a normothermic condition did not experience increased characteristics of DOMS.
Keywords: euhydration, euthermic, thermal regulation, eccentric exercise
Dehydration is a frequent problem in physically active individuals exercising at high volumes in hot ambient environments, with losses of 6% to 8% of preexercise body mass common.1,2 As little as 1% to 2% body mass loss in the heat challenges cardiovascular compensatory mechanisms for increased body temperature and reduces exercise capacity.3 Thermoregulatory shunting of blood from the core to the skin for passive heat loss and sweating provides compensatory mechanisms for cooling but may challenge both total body water and the blood volume needed to optimally perfuse the tissues of the body during exercise,3 resulting in decreased skeletal muscle perfusion.3–5
Normal skeletal muscle function is affected by altered physiologic states such as dehydration. Exercise performance decreases as less blood is available for perfusion of active skeletal muscle. Blood flow to exercising muscles is significantly reduced with dehydration due to reductions in blood pressure and perfusion pressure.4,5 Sweating is maintained by intracellular water shifting to the extracellular space, resulting in cell dehydration and adversely affecting skeletal muscle cell function.6 Dehydration negatively affects muscle performance by impeding thermal regulation, altering water movement across cell membranes, and interfering with actin-myosin cross-bridge formation.7
Delayed-onset muscle soreness (DOMS) is a clinical model of muscle damage consisting of muscular pain and other symptoms experienced 24 to 48 hours after novel or intense exercise. The signs and symptoms of DOMS include dull, diffuse pain and tenderness; stiffness; swelling; and decreased strength of the exercised muscle.8 These signs and symptoms typically last 1 to 4 days after the exercise bout.8–12 The magnitude of DOMS depends on the intensity and duration of the exercise perturbation8,10,11 as well as the physiologic condition of the individual.13,14
Eccentric exercise performed when an individual is dehydrated may exacerbate the skeletal muscle damage as a result of reduced intracellular water. Eccentric muscle activity with decreased intracellular water during dehydration has been theorized to lead to structural, contractile, and enzymatic protein denaturation.6,7 These structural and functional protein alterations might occur in addition to the initial myofiber and connective tissue damage produced by eccentric muscle tension. Rest periods before additional exercise in a thermoneutral environment have been shown to sufficiently redistribute body water and attenuate thermoregulatory and cardiorespiratory responses of dehydrated individuals.15 Ultimately, dehydration may increase the risk of DOMS in healthy individuals engaged in novel or unaccustomed physical exercise.12,16 Ostensibly, DOMS has a detrimental impact on athletic performance in that muscle endurance, strength, and power are reduced.17 The combination of the deleterious effects of dehydration and DOMS may predispose an athlete to the risk of additional injury during physical activity. A paucity of data exists concerning the effects of dehydration on eccentric-biased aerobic exercise performance and the resulting signs and symptoms of DOMS. Our purpose was to determine the effects of dehydration on the characteristics of DOMS.
METHODS
Research Design
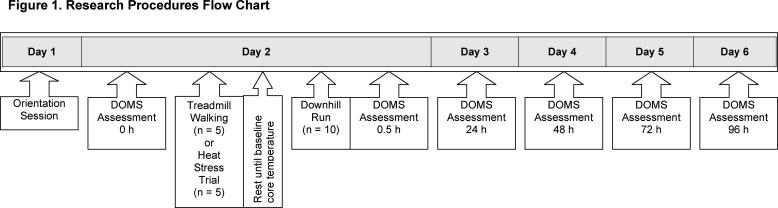
The research design consisted of a randomized group test-retest design with 2 factors (group and time) with repeated measures on time (Figure 1). Participants performed 45 minutes of treadmill walking in either a thermoneutral (euhydration) or a hot, humid (dehydration) environment. After a rest period to allow for the return to the normothermic condition, DOMS was induced with a 45-minute downhill run (−12° from horizontal). The euhydration group was allowed water ad libitum, and the dehydration group was fluid restricted. Five physiologic characteristics of DOMS were assessed preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. The study was approved by the institutional review board and was conducted at the Biokinetics Research Laboratory.
Figure 1. Research procedures flow chart. DOMS indicates delayed-onset muscle soreness.
Participants
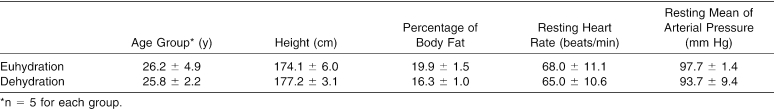
Participants were 10 healthy male students (age = 25.4 ± 3.6 years, height = 175.8 ± 7.6 cm, and body mass = 85.3 ± 3.6 kg) (Table). Males were selected to reduce the variability of hormone levels and substrate utilization between sexes during exercise. Before participating in the study, subjects completed a health history questionnaire and an informed consent form in compliance with institutional review board policies.
Age, Height, Percentage of Body Fat, Resting Heart Rate, and Resting Mean Arterial Pressure of Euhydration and Dehydration Groups (Mean ± SD).
Participants were accepted based on the absence of lower extremity eccentric training during the 6 months preceding data collection. Eccentric training was defined as downhill running, running downstairs, jumping, or weight training. Subjects maintained normal activities of daily living and refrained from initiating an exercise program during the course of the study. Additionally, they denied a history of lower limb injury or surgery, predisposing cardiovascular or cardiorespiratory conditions, or heat-related illness within 1 year preceding data collection. Participants were required to abstain from ingestion of alcohol, caffeine, and nonprescription medications for 24 hours before and during the study. They were also instructed to abstain from massaging, stretching, or otherwise treating the exercised limbs during the study.
Instruments
Visual Analog Scale
Bilateral quadriceps and overall body perceived pain were assessed using a visual analog scale (VAS). The VAS consists of a 10-cm line labeled with no soreness on the left and extremely sore on the right.18 Participants rated perceived pain by placing a slash mark on the line best corresponding to their soreness level. Perceived pain was quantified by measuring to the nearest 1 mm from the left end of the line to the slash mark.
Subjects assessed their perceived pain level using a standardized protocol. All determinations were performed twice with a blank VAS for each assessment to minimize bias from previous determinations. The averages of the 2 bilateral quadriceps determinations and the 2 overall body determinations were recorded. The VAS has been used to reliably quantify perceived pain following eccentric exercise.19–21 The VAS average intratester reliability for the current study was an intraclass correlation coefficient (ICC) (2,1) of 0.99.
Punctate Tenderness Gauge
Bilateral quadriceps punctate tenderness was assessed using the punctate tenderness gauge (PTG) (model 75 force probe gauge; Technical Products, Caldwell, NJ). Spring-loaded probes similar to the PTG have been used to reliably quantify muscular tenderness.21–25 The PTG has a 2-mm hemispheric probe attached to a force gauge. A 1.5-cm closed-cell foam stopper is attached to the end of the probe to simulate digital palpation.21–25 We used a plastic grid with 10 holes spaced 10 cm apart to standardize placement of the PTG.
Bilateral quadriceps punctate tenderness was assessed using a standardized protocol. Participants were seated on a plinth with the hips in 0° of abduction and 90° of flexion and the knees in 0° of extension. The plastic grid was positioned over the quadriceps muscle group; its distal corners were secured with self-adhesive strips over the medial and lateral femoral condyles. The gauge was inserted into each hole of the grid and depressed until the participant reported that the sensation of pressure turned to tenderness or pain. A maximum force of 14 lb (6.35 kg) was recorded and interpreted as absence of punctate muscle tenderness.21 The force required to elicit a response is inversely related to punctate muscle tenderness (ie, the more force applied, the less muscle tenderness present). Measurements were recorded to the nearest 0.25 lb (0.11 kg). Each region of the quadriceps muscle group was assessed. The vastus medialis and the rectus femoris each had 3 data collection levels: proximal, middle, and distal. The vastus lateralis had 4 data collection levels: proximal, middle proximal, middle distal, and distal. Measurements were performed twice for each data collection level and averaged for each muscle. The punctuate tenderness gauge has weights traceable to the National Bureau of Standards with a reliability of r = 0.99, P < .05 (unpublished data, Donald C. Meserlian, 1987–1991). The average intratester reliability for the gauge in the current study was an ICC (2,1) of 0.76, comparable with previously published studies.21,22
Goniometer
Goniometry has been used to assess passive range of motion (ROM) after eccentric exercise.21,26–28 Bilateral knee-flexion passive ROM was measured using a 12-in (30.48-cm) clear plastic goniometer (No. 137; Orthopedic Equipment Co, Bourbon, IN). The goniometer was marked in 1.0° increments. Bilateral knee-flexion passive ROM was assessed using a standardized protocol. Participants were positioned on a plinth prone with the hips in 0° of abduction and flexion. A folded towel was placed under each distal thigh to stabilize the femur and reduce hip rotation, flexion, and extension. The fulcrum of the goniometer was centered over the lateral femoral epicondyle, and the stationary arm of the goniometer was aligned with the greater trochanter of the femur. The movable arm of the goniometer was aligned with the lateral midline of the fibula, using the lateral malleolus as a reference.26 The starting position was 0° of knee extension. Bilateral knee-flexion passive ROM was assessed at the end range when participants either reported pain or soreness or no further movement occurred. Measurements were performed twice for each limb. The average of the 2 measurements for each limb was recorded. Passive knee-flexion ROM average intratester reliability was an ICC (2,1) of 0.99.
Anthropometric Measuring Tape
Bilateral thigh circumference was measured using a Gullick anthropometric measuring tape (Fabrication Enterprises, Everton, NY). The measuring tape has a spring-loaded gauge for improved standardization of applied tension. Bilateral thigh circumference was assessed using a standardized protocol. We measured the length of the thigh from the anterior superior iliac spine to the superior pole of the patella. Distal thigh circumference was measured at 10%, 20%, 30%, and 40% (distal, middle distal, middle proximal, and proximal sites, respectively) of the length of the thigh. Two measurements were performed at each site, and the average of the 2 measurements for each site was recorded. Limb circumference has been used to reliably assess muscle swelling after eccentric exercise.27,28 Bilateral thigh circumference intertester and average intratester reliabilities for the current study were ICC (2,1) of 0.99 and 0.99, respectively.
Isokinetic Dynamometer
Isokinetic dynamometry has been used to assess isometric strength after eccentric exercise.21,28–30 Bilateral quadriceps isometric strength was measured using the Biodex B-2000 isokinetic dynamometer (Biodex Corp, Shirley, NY), which is a multijoint testing and rehabilitation system. The dynamometer was calibrated before each data collection session in accordance with the manufacturer's guidelines.
Bilateral quadriceps isometric strength was assessed using a standardized protocol. Participants were seated on the bench with the hips and knees at 90° of flexion. They crossed their arms over their chest, and we secured straps over their arms, pelvis, and femur. The knee attachment was secured to the tibia just proximal to the talocrural joint, allowing full plantar flexion and dorsiflexion. The dynamometer axis was aligned with the joint line of the knee. The dynamometer arm was positioned at 60° (knee extension) and confirmed goniometrically. Subjects performed five 5-second submaximal isometric warm-up repetitions. Testing consisted of the participants applying as much force as possible against the immovable dynamometer arm in the direction of knee extension. Participants performed five 5-second maximal isometric repetitions for each limb with a 5-second rest between repetitions. The highest and lowest values were eliminated, and the remaining 3 values were averaged and recorded. Bilateral quadriceps isometric strength average intratester reliability was an ICC (2,1) of 0.95.
Experimental Protocols
As safety precautions, participants' rectal and skin temperatures, heart rate, mean arterial pressure, and rating of perceived exertion responses were recorded preexercise and at 15-minute intervals during treadmill walking and downhill running. Thermoregulatory response to exercise was determined via rectal probe (model 401; YSI, Yellow Springs, OH) and 4 skin thermistors (model 409A; YSI) connected to a telethermometer (model 43; YSI). Cardiovascular response to exercise was determined by measuring heart rate using the SensorMedics Max-1 12-lead electrocardiogram (Yorba Linda, CA). Participants' blood pressures were assessed standing and during exercise using a mercury sphygmomanometer (American Diagnostics, West Babylon, NY) and stethoscope (American Diagnostics). Perceived exertion was measured using the Borg Rating of Perceived Exertion Scale.31 Exercise rectal temperature and the electrocardiogram were monitored every 5 minutes.
Euhydration
The euhydration protocol consisted of participants reporting to the laboratory fully hydrated on the morning of data collection. Subjects were instructed to consume a breakfast consisting of a bagel or toast and 4 oz (118.29 mL) of orange juice. Euhydrated participants consumed water ad libitum to maintain preexercise body mass throughout exercise and data collection. To mitigate the confounding effects of exercise on DOMS, the euhydrated subjects performed 45 minutes of treadmill walking (model Q55; Quinton Instruments, Seattle, WA) in a thermoneutral environment. The treadmill walking protocol commenced with a 5-minute warm-up at 3.0 mph (4.83 kph). Treadmill speed was then increased, and the participants walked for 40 minutes at 60% to 70% of their age-predicted heart rate range. A 60-second rest was administered after every 15 minutes of walking.
Dehydration
The dehydration protocol consisted of participants reporting to the laboratory fully hydrated on the morning of data collection. The dehydration criterion was a decrease in preexercise body mass of at least 3.0%, consistent with a moderate level of dehydration.3 Subjects were dehydrated by walking on a treadmill for 45 minutes in a hot, humid environmental chamber (Tenney Engineering, Union, NJ) with fluid restriction. The treadmill was located inside the environmental chamber, which is a stainless steel vault measuring 10 × 5 × 6 m and capable of producing and maintaining temperatures up to 40°C and 85% relative humidity. The treadmill walking protocol commenced with a 5-minute warm-up at 3.0 mph (4.83 kph). Treadmill speed was then increased, and the participants walked for 40 minutes at 60% to 70% of their age-predicted heart rate range. A 60-second rest was administered after every 15 minutes of walking. Conditions inside the environmental chamber were maintained at an ambient temperature of 40.0 ± 1.7°C, a relative humidity of 66.5 ± 12.4%, and sea level ambient pressure. The first 2 environmental conditions were confirmed using a dry bulb thermometer (Bacharach, Pittsburgh, PA) and sling psychrometer (Bacharach).
Delayed-Onset Muscle Soreness Inducement
After treadmill walking, participants rested to allow rectal temperature to return to preexercise, or normothermic, levels (30 to 60 minutes) to attenuate the cardiovascular compensatory effects of exercise in the heat. We induced DOMS in all participants by having them run downhill on a treadmill elevated −12° from horizontal by placing wooden blocks under the rear of the treadmill. Downhill running was performed in a thermoneutral room and commenced with a 5-minute warm-up at 4.0 mph (6.44 kph). Treadmill speed was then increased, and subjects ran for 40 minutes at 60% of their age-predicted heart rate range.32 A 60-second rest period was administered after every 15 minutes of downhill running. At the conclusion of the data collection session, dehydrated participants were required to orally rehydrate with cool water until they returned to within 2% of their preexercise body mass.
Experimental Procedures
Participants reported to the Biokinetics Research Laboratory 24 hours before data collection for nude weighing and baseline data collection. The following day, they reported to the Laboratory and euhydrated body mass was confirmed as ±1% of baseline. Subjects were randomly assigned (n = 5) to perform treadmill walking in either a thermoneutral environment with oral rehydration to elicit the euhydration (control) condition or a hot, humid environment (40.0 ± 1.7°C, 66.5 ± 12.4% relative humidity) with fluid restriction to elicit the dehydration (experimental) condition. The criterion for dehydration was 3% body mass loss, consistent with moderate levels of dehydration. After treadmill walking, all participants rested (30 to 90 minutes) to reduce rectal temperatures to preexercise, or normothermic, levels in order to mitigate the fatiguing effect of lower extremity exercise on DOMS. They then performed 45 minutes of downhill running to induce DOMS. During all exercise sessions (treadmill walking and downhill running), heart rate, blood pressure, rating of perceived exertion, rectal temperature, and skin temperature were recorded at 15-minute intervals. After downhill running, participants were again weighed nude. At 0.5 hours postexercise, the DOMS-dependent variables were measured and recorded. Five characteristics were assessed: bilateral quadriceps and overall body perceived pain, bilateral quadriceps punctate tenderness at 3 muscle sites, bilateral knee-flexion passive ROM, bilateral thigh circumference at 4 sites, and bilateral quadriceps isometric strength. Subjects returned to the laboratory 24, 48, 72, and 96 hours postexercise for follow-up measurements of the DOMS dependent variables. At least 96 hours after exercise, the participants' body compositions were determined via hydrostatic weighing (underwater weighing scale; Chatillon Creative Health Products, Plymouth, MI) and population-specific formulae for conversion of body density to percentage of body fat.32 Subjects also performed a graded treadmill test using the SensorMedics Vmax 229 metabolic cart to determine cardiovascular fitness level. Data collection was conducted in May and July (mean maximum ambient temperature = 27.9 ± 4.8°C, National Weather Service, Mount Holly, NJ).
Data Analysis
Statistical analyses were conducted on the participants' physical characteristics and DOMS-dependent variables. Physical characteristics and hydration status were compared between groups using independent t tests. The DOMS-dependent variables were analyzed using separate 2 (group: euhydration and dehydration) × 6 (time: preexercise and 0.5, 24, 48, 72, and 96 hours postexercise) analysis of variance with repeated measures on the second factor. When applicable, bilateral data for the DOMS-dependent variables were averaged and used as the criterion measure for the data analyses. When significant interactions existed, tests of simple main effects were performed. The appropriate post hoc t test with a Bonferroni adjustment for multiple comparisons was performed to reveal the location of the significant differences. Data were analyzed using the SPSS 10.0 for Windows Statistical Package (SPSS Inc, Chicago, IL). Significance was set at P ≤ .05 for all statistical analyses.
RESULTS
Physical Characteristics
No significant differences existed between the euhydration and dehydration groups for age (t9 = 0.17, P = .87), height (t9 = −1.04, P = .33), body mass (t9 = 0.30, P = .11), percentage of body fat (t9 = 1.81, P = .12), resting heart rate (t9 = 0.44, P = .67), or resting mean arterial pressure (t9 = .94, P = .37) (see Table). However, percentage change in body mass data from preexercise to postexercise revealed significant differences between groups. The dehydration group lost significantly more body mass (t9 = 7.92, P ≤ .001) than the euhydration group. Treadmill walking in a hot, humid environment with fluid restriction resulted in a 2.7% decrease in body mass of the dehydrated participants (2.3 ± 0.1 kg). Treadmill walking in a thermoneutral environment with oral rehydration resulted in a 0.9% increase in body mass of the euhydrated participants (0.7 ± 0.4 kg).
Delayed-Onset Muscle Soreness Assessment
Bilateral Quadriceps Perceived Pain
The quadriceps perceived pain data (Figure 2) revealed a significant time main effect only. Quadriceps perceived pain was significantly higher at 0.5, 24, and 48 hours postexercise than at preexercise (t9 = −4.80, P ≤ .001; t9 = −5.48, P ≤ .001; and t9 = −6.65, P ≤ .001, respectively). Bilateral quadriceps perceived pain was also significantly higher at 24, 48, and 72 hours postexercise than at 96 hours postexercise (t9 = 4.02, P = .003; t9 = 5.95, P ≤ .001; and t9 = 4.46, P = .002, respectively) and significantly higher at 48 hours postexercise than at 72 hours postexercise (t9 = 4.24, P = .002). No interaction effects were observed for bilateral quadriceps perceived pain.
Figure 2. Perceived pain of the bilateral quadriceps muscles of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly greater at 0.5, 24, and 48 hours postexercise than at preexercise (P ≤ .001). †Significantly greater at 24, 48, and 72 hours postexercise than at 96 hours postexercise (P < .003, P ≤ .001, and P = .002, respectively). ‡Significantly greater at 48 hours postexercise than at 72 hours postexercise (P = .002). Nonsignificant for group (F1,8 = 0.087, P = .776, power = .058).
Overall Body Perceived Pain
A significant time main effect only existed for overall body perceived pain (Figure 3). Overall body perceived pain was significantly higher at 24 and 48 hours postexercise than at preexercise (t9 = −4.31, P = .002 and t9 = −4.44, P = .002, respectively). No interaction effects were observed for overall body perceived pain.
Figure 3. Perceived pain of the overall body of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly higher at 24 and 48 hours postexercise than at preexercise (P = .002). Nonsignificant for group (F1,8 = 0.024, P = .882, power = .052).
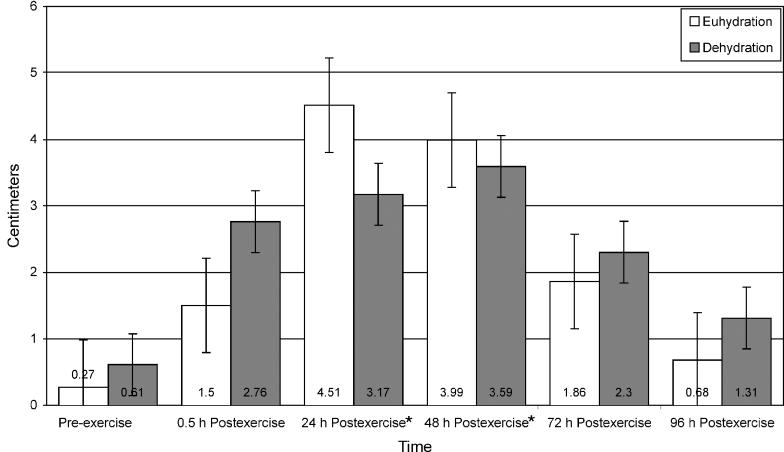
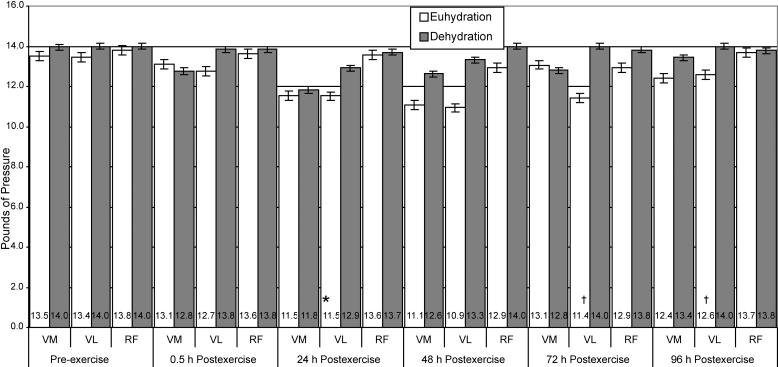
Bilateral Quadriceps Punctate Tenderness
The punctate tenderness data for the vastus medialis (Figure 4) revealed a significant time main effect only. Punctate tenderness of the vastus medialis was significantly higher at 24 hours postexercise than at preexercise and 0.5 hours postexercise for both groups (t9 = 5.36, P ≤ .001 and t9 = 4.70, P ≤ .001, respectively). Punctate tenderness data for the vastus lateralis revealed a significant interaction between group and time. Tests of simple main effects demonstrated that punctate tenderness of the vastus lateralis was 18.5% and 10.1% higher for the euhydrated participants than for the dehydrated participants at 72 and 96 hours postexercise, respectively (t9 = −3.64, P = .007 and t9 = −3.69, P = .006). No significant interaction or main effects existed for the rectus femoris.
Figure 4. Punctate tenderness of the bilateral quadriceps of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. Values are inversely related to tenderness, ie, the lower the value, the more tenderness reported. *Significantly greater (P ≤ .001) than preexercise and 0.5 hour postexercise. †Significantly higher (P ≤ .007) than dehydration group for each time. Vastus medialis nonsignificant for group (F1,8 = 0.633, P = .449, power = .108). Rectus femoris nonsignificant for time (F5,40 = 1.016, P = .421, power = .323) and group (F1,8 = 1.235, P = .299, power = .166). Muscles tested: VM indicates vastus medialis; VL, vastus lateralis; and RF, rectus femoris.
Bilateral Knee-Flexion Passive Range of Motion
Bilateral knee-flexion passive ROM (Figure 5) revealed no significant interaction or main effects.
Figure 5. Knee-flexion range of motion of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. Nonsignificant for group (F1,8 = 0.242, P = .242, power = .072) and time (F5,40 = 1.155, P = .348, power = .313).
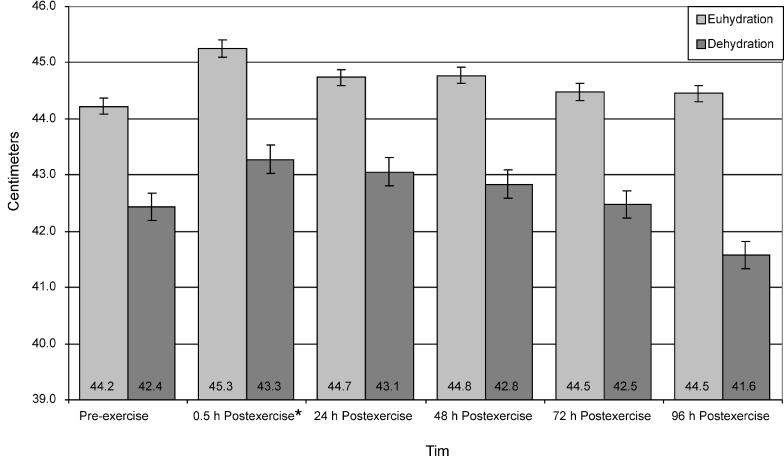
Bilateral Thigh Circumference
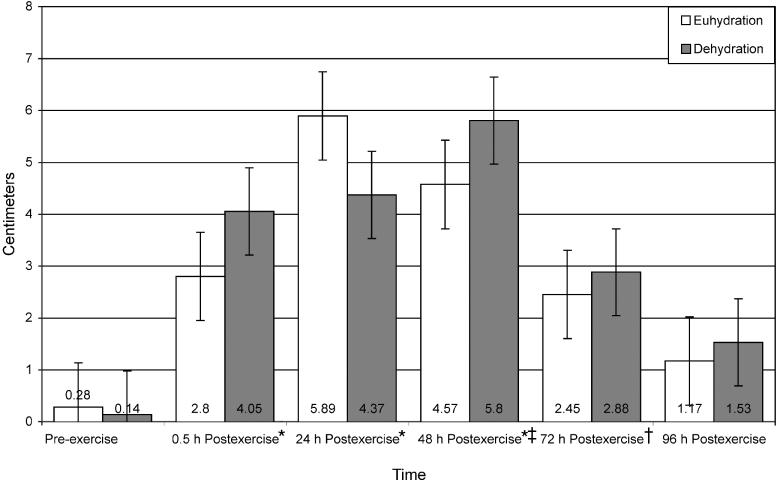
Distal bilateral thigh circumference data (Figure 6) revealed a significant time main effect only. Distal bilateral thigh circumference was significantly greater for both groups at 0.5 hours postexercise than at preexercise (t9 = −4.63, P ≤ .001). No significant interaction or main effects existed for middle distal, middle proximal, or proximal bilateral thigh circumferences.
Figure 6. Distal thigh circumference of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly greater at 0.5 hour postexercise than at preexercise (P ≤ .001). Nonsignificant for group (F1,8 = 1.711, P = .227, power = .211).
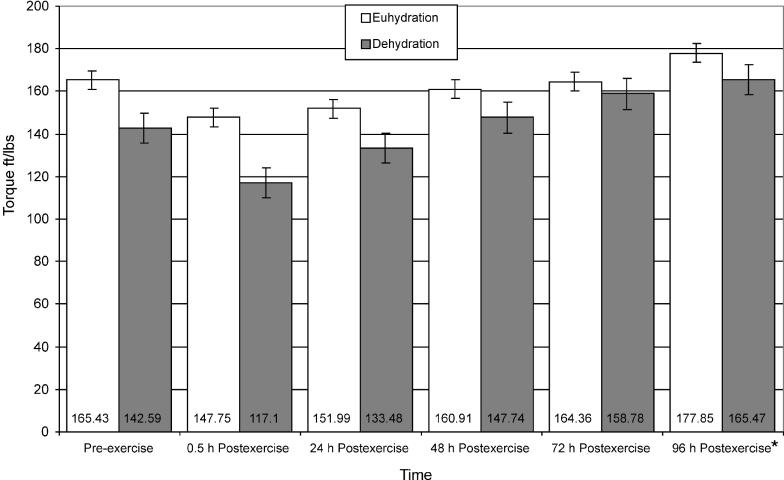
Bilateral Quadriceps Isometric Strength
Quadriceps isometric strength data (Figure 7) revealed a significant time main effect only. Quadriceps isometric strength was significantly greater at 96 hours postexercise than at 0.5 hours postexercise for both groups (t9 = −4.72, P ≤ .001). No interaction effects were observed for bilateral quadriceps isometric strength.
Figure 7. Isometric strength of euhydrated and dehydrated participants measured preexercise and at 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly greater than at 0.5 hour postexercise (P ≤ .001). Nonsignificant for group (F1,8 = 4.572, P = .065, power = .469).
DISCUSSION
We demonstrated that the downhill running exercise perturbation induced DOMS in both the euhydrated and dehydrated participants, based on increased bilateral quadriceps and overall body perceived pain and punctate tenderness of the bilateral vastus medialis muscle. All 3 variables were significantly higher at 24 hours postexercise than at preexercise. Bilateral quadriceps perceived pain was also significantly higher at 48 hours postexercise than at preexercise. Compared to its zenith at 48 hours postexercise, bilateral quadriceps perceived pain was significantly reduced at 72 and 96 hours postexercise. This classic model of DOMS has been consistently reported in the literature.10,11,33–36
Pathophysiology of Delayed-Onset Muscle Soreness
Primarily, DOMS is caused by exercise that incorporates heavy loads with passive lengthening during muscle contraction or production of eccentric muscle tension.33–35 Eccentric muscle tension is produced during lowering weight against gravity, downhill walking, and downhill running.34,35 Acute bouts of eccentric muscle tension produce microscopic lesions of the myofiber often referred to as “microdamage.”36–38 Microdamage affects the sarcolemma, sarcoplasmic reticulum, Z-lines,11,37,38 contractile components of the myofiber, and surrounding connective tissue.39–43 Skeletal muscle microdamage produced by eccentric muscle tension is subcellular and leads to concomitant events that result in the signs and symptoms associated with DOMS.44–48
The signs and symptoms of DOMS are attributed to subcellular alterations of the sarcolemma, or phospholipid membrane, as a result of skeletal muscle microdamage.49–53 The sarcolemma loses its ability to retain potassium, creatine kinase, and myoglobin, which are released into the extracellular fluid, plasma, and urine.33,36,49–53 Efflux of intramuscular ions and proteins leads to increased osmolarity of the extracellular fluid and fluid shifts out of the cell.
Increased osmolarity of the extracellular fluid enhances potassium release by cells and, in a counterproductive way, further increases extracellular fluid osmolarity and potassium concentrations. As plasma osmolarity increases, intracellular water leaves the cell because of the osmotic pressure gradient across the cell membrane. Intracellular water shifts out of the cell until the intracellular fluid osmolarity equals the extracellular fluid osmolarity. This loss of intracellular water shrinks (crenates) cells, causing the intracellular potassium concentration to rise, providing a driving force for potassium efflux from cells, further increasing extracellular fluid and plasma potassium concentration.54
During exercise, more potassium is released from skeletal muscle cells than during rest.54 Release of potassium during the recovery phase of the action potential and the ensuing plasma hyperkalemia depends on the degree of exercise. Plasma potassium concentration increases up to 0.3 mEq/L with slow walking and up to 2.0 mEq/L with heavy exercise. The extent to which these conditions alter plasma potassium concentration depends on the integrity of the sarcolemma. Under normal conditions, plasma potassium concentration is regulated by homeostatic mechanisms, such as the secretion and action of epinephrine, insulin, and aldosterone.54
Dehydration and Its Effects on Skeletal Muscle Function
During dehydration, plasma hyperosmolarity is exacerbated as water is redistributed from the intracellular to the extracellular compartments of skeletal muscle in an attempt to maintain normal blood osmolarity. Muscle proteins affected most by dehydration are those involved in electrolyte distribution across the sarcolemma (ie, sodium-potassium and calcium adenosine triphosphatases), calcium release and reuptake by the sarcoplasmic reticulum, and components of the mitochondrial respiratory chain.7 Cardiovascular compensatory mechanisms for thermoregulatory blood pooling in the skin determine tolerance to dehydration and exercise in the heat. Exercise performance decreases as less blood is available for perfusion of active skeletal muscle. Blood flow to exercising muscles is significantly reduced with dehydration due to reductions in blood pressure and perfusion pressure.55
Pain Associated With Delayed-Onset Muscle Soreness
Signs and symptoms of DOMS may be attributed to increased extracellular fluid osmolarity, which leads to swelling, edema, decreased ROM, and elevation of intramuscular pressure.56,57 Group IV sensory afferent neurons terminate in the connective tissue between myofibers and may be sensitive to the increased osmotic pressure produced by leakage of cellular contents. Activation of these neurons would result in the sensation of dull, diffuse pain associated with DOMS.58
Damage to the sarcolemma leads to the inability of the cell to modulate cellular functions and ultimately contributes to the signs and symptoms of DOMS. The reduced ability of the sarcolemma to contain and regulate cellular contents allows extracellular calcium to enter the cell, following its concentration gradient.59,60 Increased intracellular free calcium in the sarcoplasm may also result from damage or impairment of the sarcoplasmic reticulum, decreasing its ability to resequester calcium.60,61 Eccentric muscle tension produces sarcolemma damage and increased intracellular free calcium in the sarcoplasm. Increased intramuscular free calcium activates phospholipase A2,36,62 stimulating the inflammatory response associated with DOMS and promoting further degradation of cellular structures. Within 12 hours of the initial damage, cellular infiltration of neutrophils is followed by monocytes,17,51 lymphocytes, granulocytes, and interleukins.63–65 Further damage to cellular structures is caused by free radicals produced by infiltrated monocytes and calcium-stimulated proteases such as calpains.59 Eccentric muscle tension producing sarcolemma impairment leads to extensive skeletal muscle microdamage and DOMS.12,13,66 Altered osmotic pressure gradients at the tissue-capillary interface result in fluid and solute shifts in and out of the cell. Osmotic pressure gradient alterations may contribute to increased plasma osmolarity (hyperosmolarity or hemoconcentration). Fluid shifts associated with plasma hyperosmolarity adversely affect the ability of the body to regulate temperature and subsequently decrease skeletal muscle function.6
Muscle Tenderness Associated With Delayed-Onset Muscle Soreness
The increased perceived pain and punctate tenderness after downhill running were likely the result of microdamage from mechanical stress on the active muscles. Although not directly measured in this study, eccentric exercise-induced mechanical stress results in microdamage to the sarcolemma and contractile elements of the myofiber.37,38 The damaged sarcolemma allows efflux of intramuscular contents, increasing the osmolarity of the extracellular fluid.49–52 Increased osmolarity of the extracellular fluid stimulates group IV sensory afferent nerve fibers terminating near the myofiber, resulting in increased perceived pain.58 A damaged sarcolemma would have also resulted in increased intramuscular pressure, which sensitized sensory afferents terminating in the connective tissues between myofibers.36,30,41 Increased punctate tenderness is attributed to increased sensitivity to palpation as a result of increased intramuscular pressure.39,67
Of the 3 quadriceps muscles assessed for punctate tenderness, only the vastus medialis showed a significant time effect. Newham et al10,68 reported increased punctate tenderness in the vastii muscles with the rectus femoris being spared 24 to 48 hours after bench stepping.10,34,68 Schwane et al35 reported increased punctate tenderness in the quadriceps and other postural and accessory muscles of the lower extremity 24 to 48 hours after downhill running. In a related study, Cleary et al69 reported increased punctate tenderness of the vastii muscles 24 to 72 hours after downhill running and no effect on the rectus femoris. Ciccotti et al70 reported that the vastus medialis had significantly greater motor unit recruitment than the vastus lateralis and rectus femoris during the loading phase of running and downhill walking. Accordingly, the vastus medialis is an important patellofemoral joint stabilizer during ambulation, which leads to increased mechanical tension during the eccentric phase of the gait cycle and the corresponding increased punctate tenderness. The signs and symptoms of DOMS were not significantly different between the euhydrated and dehydrated groups, with one exception. Punctate tenderness of the bilateral vastus lateralis muscle was higher for the euhydrated than the dehydrated participants at 72 and 96 hours postexercise. This finding is an enigma and is counter to what was anticipated.
Swelling Associated With Delayed-Onset Muscle Soreness
Distal bilateral thigh circumference was significantly greater at 0.5 and 24 hours postexercise than at preexercise for the euhydrated and dehydrated participants. The metabolic demands of the active quadriceps muscles require increased vascular perfusion for oxygen and glucose delivery and metabolic waste removal, resulting in the increased distal thigh circumference at 0.5 hours postexercise.5,57 However, this was not the causative factor for the increase at 24 hours postexercise, which was attributed to muscle swelling associated with the inflammatory response.39,51 Smith et al63–65 reported increased circulating indices of inflammation 12 to 24 hours after downhill running, which they attributed to microdamage of the exercised myofibers. Using radionuclide-labeled leukocytes, MacIntyre et al71 confirmed the presence of inflammatory cells throughout the quadriceps 24 hours after eccentric quadriceps exercise.
Reduced passive ROM and isometric strength after an eccentric exercise perturbation have been frequently reported in the DOMS literature.30,72 Bilateral passive knee-flexion ROM was not affected by downhill running, a finding also noted by Cleary et al.69 This finding is in contrast to that of Ebbeling and Clarkson28 and Nosaka and Clarkson,73 who reported reduced passive ROM after eccentric free-weight loading of the elbow flexor muscles resulted in increased tension per motor unit area. Differences in study outcomes are attributed to differences in the mode of eccentric loading and the size of the musculature tested.
Strength Loss Associated With Delayed-Onset Muscle Soreness
We demonstrated that bilateral quadriceps isometric strength recovers by 96 hours postexercise compared with its nadir at 0.5 hours postexercise. Other researchers30,71–73 have reported reduced isometric strength up to 6 days after isokinetic eccentric quadriceps exercise. Sargeant and Dolan72 found reduced quadriceps isometric strength up to 96 hours after exhaustive downhill walking. Cleary et al69 noted that 45 minutes of downhill running resulted in significantly lower isometric strength at 0.5 hours postexercise than at preexercise and significantly higher isometric strength at 96 hours postexercise than at preexercise. The intensity and duration of the downhill running exercise perturbation and the multiple strength assessments in the current study may have been sufficient to elicit the significant strength increase. The increase is likely the result of improved neural efficiency in motor unit recruitment.74
Dehydration Alone Does Not Affect Delayed-Onset Muscle Soreness
Athletic trainers and other practitioners should be aware that treadmill walking at a moderate intensity in a hot, humid environment with fluid restriction resulted in a 2.7% body mass reduction. Researchers2 have demonstrated that body mass losses as low as 1% to 2% compromise physical performance as a result of cardiovascular compensations for heat load. The signs and symptoms of DOMS after an eccentric exercise perturbation were not exacerbated by moderate dehydration of 2.7% body mass with rest and a return to the normothermic condition. Although the goal had been to dehydrate the participants to 3%, this was not accomplished due to the participants' negative physiologic responses to the dehydration protocol.
Heat load in the current study was attenuated in the dehydrated participants during the 30- to 90-minute rest period before downhill running. Heat load indices were reduced before the downhill run, as demonstrated by no significant differences between the euhydrated and dehydrated participants' thermoregulatory, cardiorespiratory, and rating of perceived exertion responses. In our study, the effects of lower extremity exercise and moderate dehydration were attenuated by rest. The attenuated effects may not have been severe enough to elicit deficits in muscle function.
We acknowledge the limitations of the current study. Power for nonsignificant findings between groups ranged from .052 to .469, which may be attributed to our small sample size or a less than anticipated effect size. Acclimation probably was not a factor, as the average maximum ambient temperature during the data collection period was 27.9 ± 4.8°C and participants did become moderately dehydrated. It is also possible that downhill running in a hot, humid environment or dehydration levels greater than 2.7% may elicit a threshold sufficient to exacerbate the signs and symptoms of DOMS.
Water lost via sweating is thought to be replaced by movement of intracellular water from inactive tissues to defend plasma volume and active skeletal muscle during dehydration. This outcome has been substantiated in prior research.15,75 Greiwe et al76 reported that dehydration of 3.8% body mass and attenuation of heat load indices by a 3-hour rest had no significant effect on isometric quadriceps muscle strength. Nielsen et al5 noted that 60 minutes of uphill walking in the heat without dehydration increased intramuscular temperatures but did not affect muscle blood flow. Moderate dehydration without a concomitant heat load does not exacerbate eccentric exercise-induced skeletal muscle microdamage.
Practitioners should be aware that dehydration combined with heat load from the environment and from metabolic heat generated during eccentric exercise may potentiate the signs and symptoms of DOMS in dehydrated participants and warrants further investigation. Cleary et al69 demonstrated more perceived pain and punctate quadriceps tenderness 24 to 72 hours after 45 minutes of downhill running in hyperthermic participants who were 3.3% dehydrated than in hyperthermic participants who were euhydrated. Eccentric exercise-induced microdamage and the concomitant signs and symptoms of DOMS may be exacerbated by reduced tissue perfusion, heat load, metabolic heat, reduced muscle strength, and denaturation of structural and functional proteins.7
We demonstrated that a treadmill walking protocol in a hot, humid environment resulted in a 2.7% reduction in body mass and the downhill running eccentric exercise perturbation resulted in a classic pattern of the signs and symptoms of DOMS. Athletes participating with DOMS may be susceptible to further injury as a result of pain, swelling, and decreased muscle function. Although dehydration to 2.7% of preexercise body mass with rest and attenuation of heat load had no deleterious effects on the signs and symptoms of DOMS, the extent to which this is true at dehydration levels greater than 2.7% is unknown and warrants further study.
REFERENCES
- Sawka MN, Knowlton RG, Critz JB. Thermal and circulatory responses to repeated bouts of prolonged running. Med Sci Sports. 1979;11:177–180. [PubMed] [Google Scholar]
- Sawka MN, Latzka WA, Matott RP, Montain SJ. Hydration effects on temperature regulation. Int J Sports Med. 1998;19:S108–S110. doi: 10.1055/s-2007-971971. (suppl) [DOI] [PubMed] [Google Scholar]
- Haynes EM, Wells CL. Environment and Human Performance. Champaign, IL: Human Kinetics; 1986.
- Gonzalez-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol. 1998;513:895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Sawka MN. Physiological consequences of hypohydration: exercise performance and thermal regulation. Med Sci Sports Exerc. 1992;24:657–670. [PubMed] [Google Scholar]
- Hargreaves M, Febbraio M. Limits to exercise performance in the heat. Int J Sports Med. 1998;19:S115–S116. doi: 10.1055/s-2007-971973. (suppl) [DOI] [PubMed] [Google Scholar]
- Fitzgerald GK, Rothstein JM, Mayhew TP, Lamb RL. Exercise-induced muscle soreness after concentric and eccentric isokinetic contractions. Phys Ther. 1991;7:505–513. doi: 10.1093/ptj/71.7.505. [DOI] [PubMed] [Google Scholar]
- Abraham WM. Factors in delayed muscle soreness. Med Sci Sports. 1977;9:11–20. [PubMed] [Google Scholar]
- Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Kuipers H. Exercise-induced muscle damage. Int J Sports Med. 1994;15:132–135. doi: 10.1055/s-2007-1021034. [DOI] [PubMed] [Google Scholar]
- Milne CJ. Rhabdomyolysis, myoglobinuria, and exercise. Sports Med. 1988;6:93–106. doi: 10.2165/00007256-198806020-00004. [DOI] [PubMed] [Google Scholar]
- Kenney WL. Heat flux and storage in hot environments. Int J Sports Med. 1998;19:S92–S95. doi: 10.1055/s-2007-971966. (suppl) [DOI] [PubMed] [Google Scholar]
- Senay LC., Jr. Effects of exercise in the heat on body fluid distribution. Med Sci Sports. 1979;11:42–48. [PubMed] [Google Scholar]
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscle soreness: a brief review. Med Sci Sports Exerc. 1984;16:529–538. [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23:542–551. [PubMed] [Google Scholar]
- Melzack R. The short form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Flandry F, Hunt JP, Terry GC, Hughston JC. Analysis of subjective knee complaints using visual analog scales. Am J Sports Med. 1991;19:112–118. doi: 10.1177/036354659101900204. [DOI] [PubMed] [Google Scholar]
- Mattacola CG, Perrin DH, Gansneder BM, Allen JD, Mickey CA. A comparison of visual analog and graphic pain rating scales following delayed onset muscle soreness. J Sport Rehabil. 1997;6:38–646. [Google Scholar]
- Gulick DT, Kimura IF, Sitler M, Paolone A, Kelly JD., IV. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31:145–152. [PMC free article] [PubMed] [Google Scholar]
- Cleary MA, Kimura IF, Sitler MR, Kendrick ZV. Temporal pattern of the repeated bout effect of eccentric exercise on delayed-onset muscle soreness. J Athl Train. 2002;37:32–36. [PMC free article] [PubMed] [Google Scholar]
- Cott A, Parkinson W, Bell MJ. Interrater reliability of tender point criterion for fibromyalgia. J Rheumatol. 1992;19:1955–1959. et al. [PubMed] [Google Scholar]
- McCarty DJ, Jr, Gatter RA, Phelps P. A dolorimeter for quantification of articular tenderness. Arthritis Rheumatol. 1965;8:551–559. doi: 10.1002/art.1780080409. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Mills KR, Newham DJ. Measurement of severity and distribution of experimental muscle tenderness. J Physiol (Lond) 1981;317:1P–2P. [Google Scholar]
- Norkin CC, White DC. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: FA Davis; 1985.
- Chelboun GS, Howell JN, Conatser RR, Giesey JJ. Relationship between muscle swelling and stiffness after eccentric exercise. Med Sci Sports Exerc. 1998;30:529–535. doi: 10.1097/00005768-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med. 1998;7:207–234. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- Saxton JM, Donnelly AE. Light concentric exercise during recovery from exercise-induced muscle damage. Int J Sports Med. 1995;16:347–351. doi: 10.1055/s-2007-973018. [DOI] [PubMed] [Google Scholar]
- Golden CL, Dudley GA. Strength after bouts of eccentric or concentric contractions. Med Sci Sports Exerc. 1992;24:926–933. [PubMed] [Google Scholar]
- Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Heyward VH. Assessing body composition. In: Advanced Fitness Assessment and Exercise Prescription. Champaign, IL: Human Kinetics; 1997:145–172.
- Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Blood markers of delayed onset muscular soreness with downhill treadmill running. Med Sci Sports Exerc. 1981;13:80. [PubMed] [Google Scholar]
- Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc. 1983;15:51–56. [PubMed] [Google Scholar]
- Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22:429–435. [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Round JM, Tolfree SE. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden J, Seger J, Ekblom B. Sublethal muscle fibre injuries after high-tension anaerobic exercise. Eur J Appl Physiol Occup Physiol. 1988;57:360–368. doi: 10.1007/BF00635996. [DOI] [PubMed] [Google Scholar]
- Friden J, Sfakianos PN, Hargens AR, Akeson WH. Residual muscular swelling after repetitive eccentric contractions. J Orthop Res. 1988;6:493–498. doi: 10.1002/jor.1100060404. [DOI] [PubMed] [Google Scholar]
- Francis KT. Delayed muscle soreness: a review. J Orthop Sports Phys Ther. 1983;5:10–13. doi: 10.2519/jospt.1983.5.1.10. [DOI] [PubMed] [Google Scholar]
- Newham DJ. The consequences of eccentric contractions and their relationship to delayed onset muscle pain. Eur J Appl Physiol Occup Physiol. 1988;57:353–359. doi: 10.1007/BF00635995. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74:520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Clarkson PM, Fritz VK, Evans WJ. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol. 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Duarte JA, Appell HJ, Carvalho F, Bastos ML, Soares JM. Endothelium-derived oxidative stress may contribute to exercise induced muscle damage. Int J Sports Med. 1993;14:440–443. doi: 10.1055/s-2007-1021207. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Meredith CN, Cannon JG. Metabolic changes following eccentric exercise in trained and untrained men. J Appl Physiol. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. et al. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc. 1992;24:521–530. [PubMed] [Google Scholar]
- Friden J, Lieber RL, Thornell LE. Subtle indications of muscle damage following eccentric contractions. Acta Physiol Scand. 1991;142:523–524. doi: 10.1111/j.1748-1716.1991.tb09189.x. [DOI] [PubMed] [Google Scholar]
- McCully KK. Exercise-induced injury to skeletal muscle. Fed Proc. 1986;45:2933–2936. [PubMed] [Google Scholar]
- Apple FS, Rogers MA, Sherman WM, Ivy JL. Comparison of elevated serum creatine kinase MB activities post marathon and post myocardial infarction [abstract] Med Sci Sports Exerc. 1983;15:165. [Google Scholar]
- Apple FS, Hellsten Y, Clarkson PM. Early detection of skeletal muscle injury by assay of creatine kinase MM isoforms in serum after acute exercise. Clin Chem. 1988;34:1102–1104. [PubMed] [Google Scholar]
- Franklin ME, Currier DP, Franklin RC. The effect of one session of muscle soreness-inducing weight lifting exercise on WBC count, serum creatine kinase, and plasma volume. J Orthop Sports Phys Ther. 1991;13:316–320. doi: 10.2519/jospt.1991.13.6.316. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, Clarkson PM. Creatine kinase release and clearance using MM variants following repeated bouts of eccentric exercise. Med Sci Sports Exerc. 1998;30:1059–1065. doi: 10.1097/00005768-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Komulainen J, Kytola J, Vihko V. Running-induced muscle injury and myocellular enzyme release in rats. J Appl Physiol. 1994;77:2299–2304. doi: 10.1152/jappl.1994.77.5.2299. [DOI] [PubMed] [Google Scholar]
- Berne RM, Levy MN. Principles of Physiology. 2nd ed. St Louis, MO: CV Mosby; 1996.
- Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- Brendstrup P. Late edema after muscular exercise. Arch Phys Med Rehabil. 1962;43:401–405. [PubMed] [Google Scholar]
- Howell JN, Chila AG, Ford G, David D, Gates T. An electromyographic study of elbow motion during postexercise muscle soreness. J Appl Physiol. 1985;58:1713–1718. doi: 10.1152/jappl.1985.58.5.1713. [DOI] [PubMed] [Google Scholar]
- Mense S, Schmidt RF. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974;72:305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- Duan C, Delp MD, Hayes DA, Delp PD, Armstrong RB. Rat skeletal muscle mitochondria calcium and injury from downhill walking. J Appl Physiol. 1990;68:1241–1251. doi: 10.1152/jappl.1990.68.3.1241. [DOI] [PubMed] [Google Scholar]
- Byrd SK. Alterations in the sarcoplasmic reticulum: a possible link to exercise-induced muscle damage. Med Sci Sports Exerc. 1992;24:531–536. [PubMed] [Google Scholar]
- Warren GL, Hayes DA, Lowe DA, Armstrong RB. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol. 1993;464:457–475. doi: 10.1113/jphysiol.1993.sp019645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar R, Nguyen JT, Brooke MH. Effects of calcium channel antagonists on the release of prostaglandin E2 from metabolically stressed muscle. Biochem Pharmacol. 1994;48:371–374. doi: 10.1016/0006-2952(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Smith LL, Herbert WG, Hinkle DE. Neutrophilia: a potential sign of inflammation associated with delayed muscle soreness [abstract] Med Sci Sports Exerc. 1986;18:43. [Google Scholar]
- Smith LL, Bond JA, Holbert D. Differential white cell count after two bouts of downhill running. Int J Sports Med. 1998;19:432–437. doi: 10.1055/s-2007-971941. et al. [DOI] [PubMed] [Google Scholar]
- Smith LL, McCammon M, Smith S, Chamness M, Israel RG, O'Brien KF. White blood cell response to uphill walking and downhill jogging at similar metabolic loads. Eur J Appl Physiol Occup Physiol. 1989;58:833–837. doi: 10.1007/BF02332215. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membrane: role in exercise-induced damage. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- Bobbert MF, Hollander AP, Huijing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18:75–81. [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Tolfree SE, Edwards RH. Skeletal muscle damage: a study of isotope uptake, enzyme efflux, and pain after stepping. Eur J Appl Physiol Occup Physiol. 1986;55:106–112. doi: 10.1007/BF00422903. [DOI] [PubMed] [Google Scholar]
- Cleary MA, Sweeney LA, Sitler MR, Kendrick ZV. Dehydration and symptoms of delayed-onset muscle soreness in hyperthermic males. J Athl Train. 2005;40:288–297. [PMC free article] [PubMed] [Google Scholar]
- Ciccotti MG, Kerlan RK, Perry J, Pink M. An electromyographic analysis of the knee during functional activities, I: the normal profile. Am J Sports Med. 1994;22:645–650. doi: 10.1177/036354659402200512. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid DC, Lyster DM, Szasz IJ, McKenzie DC. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol. 1996;80:1006–1013. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Dolan P. Human muscle function following prolonged eccentric exercise. Eur J Appl Physiol Occup Physiol. 1987;56:704–711. doi: 10.1007/BF00424814. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM. Influence of previous concentric exercise on eccentric exercise-induced muscle damage. J Sports Sci. 1997;15:477–483. doi: 10.1080/026404197367119. [DOI] [PubMed] [Google Scholar]
- Sale DG. Neural adaptation to resistance training [abstract] Med Sci Sports Exerc. 1998;30:S135. doi: 10.1249/00005768-198810001-00009. (suppl) [DOI] [PubMed] [Google Scholar]
- Costill DL, Cote R, Fink W. Muscle water and electrolytes following varied levels of dehydration in man. J Appl Physiol. 1976;40:6–11. doi: 10.1152/jappl.1976.40.1.6. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Staffey KS, Melrose DR, Narve MD, Knowlton RG. Effects of dehydration on isometric muscular strength and endurance. Med Sci Sports Exerc. 1998;30:284–288. doi: 10.1097/00005768-199802000-00017. [DOI] [PubMed] [Google Scholar]