Abstract
Context: Theoretically, the risk of compartment syndrome is increased during creatine monohydrate (CrM) supplementation because of intracellular fluid retention in muscle cells and the overall increased size of the muscle tissue. Whether this change in intracellular fluid is associated with an increase in anterior compartment pressure in the lower leg when subjects are under thermal stress is unknown.
Objective: To assess the influence of CrM on the resting and postexercise anterior compartment pressure of the lower leg in mildly to moderately dehydrated males exercising in the heat.
Design: Double-blind, randomized, crossover design.
Setting: Human Performance Laboratory.
Patients or Other Participants: Eleven well-trained, non– heat-acclimated, healthy males (age = 22 ± 2 years, height = 181.1 ± 7 cm, mass = 78.4 ± 4.2 kg, V̇o2max = 50.5 ± 3.4 mL·kg−1·min−1).
Intervention(s): Subjects were supplemented with 21.6 g/d of CrM or placebo for 7 days. On day 7, they performed 2 hours of submaximal exercise, alternating 30 minutes of walking with 30 minutes of cycling in the heat, resulting in approximately 2% dehydration. This was followed by an 80-minute heat tolerance test (temperature = 33.5 ± 0.5°C, humidity = 41.0 ± 12%), which included 12 repetitions of a 3-minute walk (pace = 4.0 ± 0.1 miles/h, intensity = 37.1 ± 6.1% V̇o2max) alternating with a 1-minute, high-intensity run (pace = 11.8 ± 0.4 miles/h, intensity = 115.0 ± 5.6% V̇o2max), resulting in an additional 2% decrease in body weight.
Main Outcome Measures: Before supplementation and on day 7 of supplementation, anterior compartment pressure was measured at rest, after dehydration, and at 1, 3, 5, 10, 15, and 60 minutes after the heat tolerance test. Analysis of variance with repeated measures was calculated to compare differences within the trials and time points and to identify any interaction between trial and time.
Results: The CrM intake was associated with an increase in body weight (P < .05). A moderate effect size was noted for compartment pressures between the trials for the differences between predehydration and postdehydration (η2 = 0.414). This effect diminished substantially by 3 minutes after the heat tolerance test. Compared with the placebo trial, the change in anterior compartment pressure from rest to dehydration was greater, as was the change from rest to 1 minute after the heat tolerance test (P < .05) during the CrM trial.
Conclusions: A 7-day loading dose of CrM increased anterior compartment pressures after dehydration and immediately after the heat tolerance tests, but the changes did not induce symptoms and the pressure changes were transient.
Keywords: ergogenic aids, compartment syndrome, thermoregulation
INTRODUCTION
Prevalent use of creatine monohydrate (CrM) as a potential ergogenic aid among all levels of athletes has occurred since it was demonstrated to aid in the production of adenosine triphosphate, which is important for short-term strength and power activities.1–10 As with any supplement, questions concerning the health effects of CrM have been raised. To date, the only consistently documented side effect of CrM supplementation has been an increase in body mass.1–13 Because of this consistent finding, CrM supplementation has been used with the primary goal of increasing body mass. Several other anecdotal effects of CrM supplementation have been reported in athletic populations, including muscle cramps, gastrointestinal disturbances, increased muscle injuries, and compartment syndrome of the quadriceps or lower leg.2–17
The increase in body mass reported in the early phase of CrM supplementation has not been attributed to a significant increase in total muscle mass.3,5,6,10,18 Rather, the likely mechanism is an increase in intracellular water retention.3,5,6,10,18 Creatine monohydrate is a powerful osmolyte that increases the water within the cell.19,20 Preliminary research has shown that CrM use alters the distribution of fluids within the body, with a 2% to 3% increase in intracellular fluid.19,20
Theoretically, the risk of compartment syndrome is increased while supplementing with CrM because of the intracellular fluid retention in the muscle cell and increased overall size of the muscle tissue.14–17 Under normal conditions without CrM supplementation, exercise produces a 20% increase in muscle volume, stemming from an increase in regional blood volume and increased transcapillary filtration of intravascular fluid beyond the dispersal ability of the lymphatic flow.21 If this normal increase is coupled with the potentially dangerous entrapment of intracellular water in an osteofascial compartment, serious medical concerns may exist for individuals supplementing with CrM.
Schroder et al16 documented that oral CrM supplementation raised resting and postexercise anterior compartment pressure (ACP) and delayed the return of postexercise ACP to resting values in the lower leg after 6 and 34 days of CrM supplementation compared with a placebo group. Potteiger et al17 also demonstrated an increase in ACP in the lower leg at rest and following exercise after 7 and 35 days of CrM supplementation, with pressures that began to return to normal after 28 days of no supplementation. Multiple authors15–17 have also demonstrated that select individuals reach exceptionally high resting and postexercise ACPs, which are classified as clinical values for the diagnosis of compartment syndrome. These people exhibit the signs and symptoms of anterior compartment syndrome of the lower leg, including aching, cramping, and burning pain and/or tightness over the area of the anterior compartment of the lower leg.
In 2000, the American College of Sports Medicine issued a recommendation to avoid CrM supplementation in hot environments, despite the lack of direct evidence of a link between the development of dehydration or heat illnesses and CrM supplementation.4 Since that publication, no authors have examined whether a change in intracellur fluid, attributed to CrM supplementation, is associated with an increase in ACP in the lower leg in the presence of thermal stress. Thus, research is needed to determine whether such fluid serves as a beneficial source of stored water to aid the dehydrated person, or whether this fluid is a potential hazard of trapped water and leads to increased compartment pressure.
Our purpose was to assess the influence of dehydration (2% to 5% reduction in body weight) and exercise in the heat on resting and postexercise ACP measures of the lower leg in trained individuals ingesting CrM. Ours is the first study to investigate the collective effects of CrM use, prolonged and intense exercise, high temperature, and dehydration on ACP measurements of the lower leg.
METHODS
Subjects
Eleven well-trained, non–heat-acclimated male subjects (age = 21 ± 2 years, height = 181.1 ± 7 cm, mass = 78.4 ± 4.2 kg, body fat = 9.4 ± 2.5%, V̇o2peak = 50.5 ± 3.3 mL·kg−1·min−1) completed the 2 trials in this study. The testing protocol and informed consent information were approved by the University of Connecticut Institutional Review Board for Studies Involving Human Subjects. All subjects attended a briefing meeting before experimentation to ensure an understanding of the testing protocol and the benefits and risks of the study and signed the informed consent statement. All subjects also completed a medical and exercise history questionnaire. Screening information was obtained to ensure that subjects met the following criteria: (1) no chronic health problems, (2) no previous history of heat illness, (3) no history of cardiovascular, metabolic, or respiratory disease, (4) no history of CrM use in the past year, and (5) no history of lower leg conditions involving the neurologic or vascular structures. Subjects were paid for their participation in this study.
Experimental Design
We used a double-blind, randomized, crossover, placebo-controlled study design. The study consisted of 2 identical sessions with the exception of the treatment. A random selection of 6 subjects began with trial A, whereas 5 subjects started with trial B. The 2 trials were (A) 21.6 g of placebo (P) (18 capsules) per day for 7 days, and (B) 21.6 g of CrM (18 capsules/day; Mega Creatine Fuel; Twinlab Laboratories, Inc, Ronkonkoma, NY) per day for 7 days. On the morning of day 7 of each experimental trial, the subjects reported to the Human Performance Laboratory to undergo a 2-part exercise protocol in the heat: (1) a submaximal aerobic exercise regimen (DHY) designed to result in an approximate 2% decline in body mass, and (2) an intense anaerobic exercise protocol (heat tolerance test [HTT]) designed to result in an approximate additional 2% decline in body weight.
Predehydration
On day 7, the subject reported in a well-hydrated state and not having eaten for 12 hours. Hydration status was verified via urine specific gravity. The lower leg was then prepared and anesthetized, and the indwelling catheter was placed in the anterior compartment of the lower leg. Next, the subject received a standard breakfast of a plain bagel with cream cheese, a banana, apple juice, and the treatment dose. After breakfast, each subject positioned a rectal thermistor and stood in the environmental chamber (temperature = 33.5 ± 0.6°C, humidity = 41 ± 12%) for 30 minutes. Heat and humidity in the environmental chamber were set at levels commonly experienced by athletes, especially during early-season 2- or 3-a-day exercise sessions in August throughout the United States in preparation for high school and collegiate sport seasons.22 During the 30 minutes of equilibration, pre-DHY measurements were recorded, including ACP, body mass, blood pressure, and perceptual measures. The body mass recorded at that time was the starting point for the DHY procedure. The ACP measures were taken to provide a resting pre-DHY measurement.
Dehydration
After the equilibration period, the subject began the DHY procedure. This involved alternating 30 minutes of walking (pace = 6.6 ± 0.32 kph, intensity = 37.1 ± 6.1% V̇o2max) on a treadmill with 30 minutes of stationary cycling (at a similar relative intensity) in the heat (temperature = 33.5 ± 0.5°C, humidity = 41 ± 12%) for a total of 120 minutes, resulting in 1.9 ± 0.5% DHY. A 120-minute exercise session to elicit approximately a 2% reduction in body weight was chosen because this is a common level of dehydration that an athlete would have beginning an exercise session, especially during multiple exercise sessions on the same day or on successive days of intense exercise in the heat.22
After the 120 minutes of exercise, the subject sat quietly in the chamber for 60 minutes and consumed a snack of 1 Gatorade Sports Bar (Chicago, IL) and 200 mL of water along with the treatment (CrM or P) dose. After ingesting the snack, the subject stood in the environmental chamber for 30 minutes and pre-HTT measurements were recorded, including ACP, body mass, blood pressure, and perceptual measures. The ACP measures were taken to provide a DHY measurement (post-DHY).
Heat Tolerance Test
After the DHY, snack, and equilibration, the subject then performed an 80-minute run on a treadmill. Specifically, the HTT included 12 repetitions, alternating a 3-minute walk (pace = 4.0 ± 0.1 miles·h−1, intensity = 37.1 ± 6.1% V̇o2max) with a high-intensity, 1-minute sprint (pace = 6.6 ± 0.32 kph, intensity = 115.0 ± 5.6% V̇o2max), resulting in an additional 2% decline in body weight. We chose an 80-minute HTT because this is a typical length of a practice session or game in numerous sports. The exercise was terminated if the subject stopped because of volitional exhaustion, had a rectal temperature greater than or equal to 40.0°C, had a heart rate ≥ 185 beats·min−1 for 5 consecutive minutes, exhibited signs of heat intolerance, or could not maintain a normal running cadence.
After the HTT, ACP measures were recorded at 1 (1 post-HTT), 3 (3 post-HTT), 5 (5 post-HTT), 10 (10 post-HTT), 15 (15 post-HTT), and 60 (60 post-HTT) minutes postexercise. After the 5-minute postexercise ACP measurement, the subject stood for the remaining 55 minutes in the thermoneutral environment and rehydrated with 1 L of water within 10 minutes after leaving the chamber. Body weight and many psychological factors, including thermal sensation, the Environmental Symptoms Questionnaire, and a medical signs and symptoms checklist were also examined before, during, and after the HTT. After standing for 60 minutes, the subject was then allowed to sit or lie down, and the indwelling catheter was removed. The subject was then given an additional 1 L of water and another Gatorade Sports Bar and was monitored for 30 to 60 minutes.
Creatine Monohydrate Supplementation
We chose a 21.6 g/d dosage of CrM because athletes typically use about 20 to 25 g/d for 7 to 10 days during the loading phase of CrM supplementation.2–4,19 On days 1–7, subjects consumed 7.2 g of either P or CrM in identical tablet form at breakfast, lunch, and dinner. After the HTT, they began a washout period. The mean washout period was 54 ± 10 days. Subjects were instructed to not consume any alcohol or drugs for the 7 days of each experimental iteration. We also instructed subjects to maintain their normal fitness training regimens throughout the study and during the washout period. Each subject then began the second experimental trial, which was an exact duplicate of the first except that the subject received the treatment opposite to that received in the first experimental trial.
Anterior Compartment Pressure Measurement
Anterior compartment pressure was measured using a Stryker Intracompartmental Pressure Monitor System (Stryker Instruments, Kalamazoo, MI). Before catheter insertion, we calibrated the Stryker System according to the guidelines provided by the manufacturer.
The lower leg was first prepared by shaving from the knee to the middle of the lower leg, and the insertion site 6 cm distal to the inferior aspect of the tibial tuberosity and 3.5 cm lateral to the anterior tibial spine was marked. While the subject was lying supine with the great toe pointed vertically, the skin over the tibialis anterior muscle was cleaned with alcohol and anesthetized with 5 mL of 2% lidocaine without epinephrine. The insertion area was then sterilized and cleaned with a Betadine solution (Purdue Frederick Co, Norwalk, CT). An indwelling catheter was next inserted by piercing the skin and fascia at an angle of approximately 45° with a breakaway needle, which was advanced 2 to 3 cm into the tibialis anterior muscle belly. After the catheter was in place, the needle was removed, and the catheter was capped with a stopcock device to ensure a closed system and taped into place to allow the subject to exercise. Excessive tape was removed before measurements were taken to prevent artificial increases in the ACP. At several points in the trial, the catheter was flushed with a heparin solution to prevent a blood clot from forming in the catheter.
For a measurement, the subject was placed in a standing position with all his weight on the contralateral leg and buttocks leaning against the rail of the treadmill. The subject's leg was placed on a chair in front of him with his knee extended fully and his ankle dorsiflexed to 80°. When the anterior tibialis muscle was felt to be relaxed on palpation to avoid excessive pressure, the cap was removed from the stopcock, and the Stryker System was connected to the catheter. The system was flushed with less than 3 mL of the heparin and saline solution, and when the pressure equalized, the digital measurement was recorded.
Statistical Analysis
Data were reported as mean ± SD. Analyses of variance with repeated measures were used to compare differences within the 2 trials and the time points and any interaction between time and trial. For the ACP measures, the within-subjects factors were trial with 2 levels (CrM, P) and time with 8 levels (pre-DHY, post-DHY, 1 post-HTT, 3 post-HTT, 5 post-HTT, 10 post-HTT, 15 post-HTT, 60 post-HTT). For the body mass and resting blood pressure measures, the within-subjects factors were trial with 2 levels (CrM, P) and time with 2 levels (day 1, day 7). We calculated paired-sample t tests using a Bonferroni correction to follow up significant trial effects, time effects, and interactions. Significance was set at the .05 level of confidence. All statistics were run using SPSS (version 10.0 for Windows; SPSS Inc, Chicago, IL).
RESULTS
Body Mass and Blood Pressure Measurements
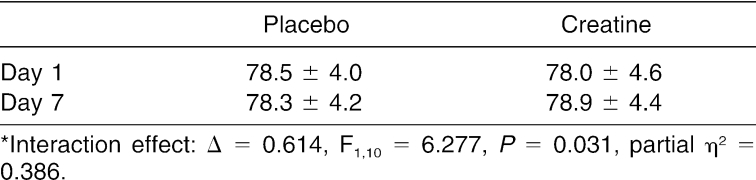
We noted a significant interaction for body mass (Δ = 0.614, F1,10 = 6.277, P = .031, partial η2 = 0.386) (Table 1). The mean body mass for the CrM group increased about 1 kg (by 0.88 ± 1.08 kg), whereas the P group's body mass decreased slightly (by 0.17 ± 0.83 kg) from day 1 to day 7. We failed to find interaction effects or time or trial main effects in resting blood pressure measurements between the trials for resting systolic or diastolic blood pressure (P > .05).
Table 1. Effect of Placebo and Creatine Supplementation on Body Weight (kg)*.
Anterior Compartment Pressure Measurements
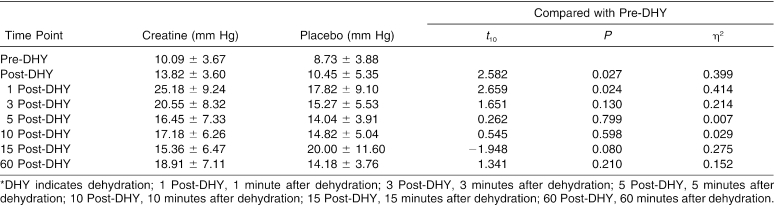
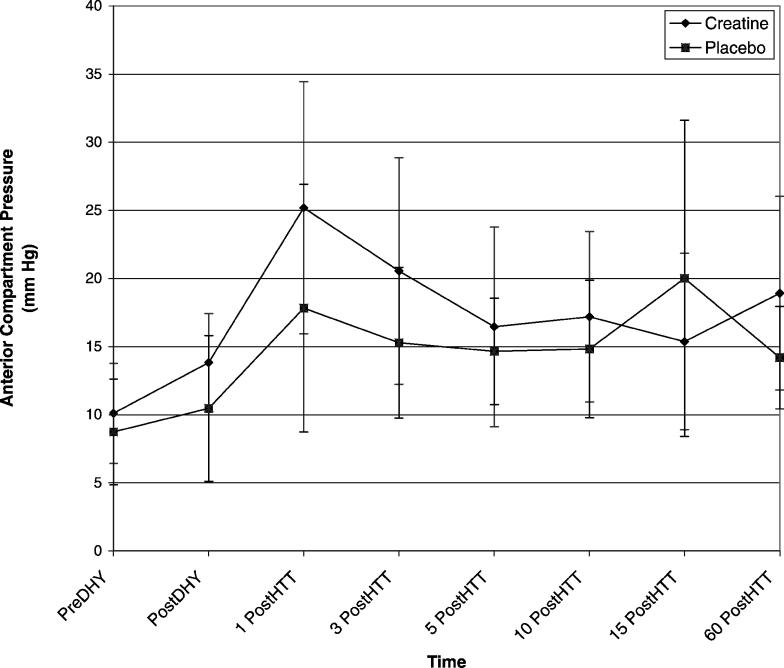
The trial × time interaction effect for ACP was significant (Δ = 0.016, F7,4 = 34.749, P = .002). We conducted 7 paired-samples t tests to follow up on the significant interaction. Differences in ACP measurements between the pre-DHY time point and each of the 6 following time points were not significantly different between the trials after adjustments were made. However, the data demonstrated a moderate effect size between the trials for the differences between pre-DHY and post-DHY (Table 2) and pre-DHY and 1 post-HTT (Table 2). A small effect size was demonstrated between the trials for pre-DHY when paired with 3 post-HTT (Table 2), 15 post-HTT (Table 2), and 60 DHY (Figure).
Table 2. Anterior Compartment Pressures (Mean ± SD)*.
Mean anterior compartment pressures for each trial. DHY indicates dehydration; 1 PostDHY, 1 minute after dehydration; 3 PostDHY, 3 minutes after dehydration; 5 PostDHY, 5 minutes after dehydration; 10 PostDHY, 10 minutes after dehydration; 15 PostDHY, 15 minutes after dehydration; and 60 PostDHY, 60 minutes after dehydration.
Adverse Physical Effects
Of the 11 subjects, 10 completed the entire 80-minute HTT exercise protocol at the specified relative intensities based on oxygen consumption collected during preexperimental testing. One subject's test was terminated at minute 60 of the HTT protocol because of a rectal temperature greater than 40.0°C. No adverse physical effects were reported for any subjects in either trial for the supplementation protocol or exercise in the heat. Specifically, no subject complained of symptoms resembling anterior compartment syndrome. This included no reports of lower extremity aching, cramping, burning pain, or tightness in the lower leg or anterior compartment, which was determined by review of a questionnaire and a medical signs and symptoms checklist that subjects completed before and after the DHY and HTT.
DISCUSSION
Our results show that short-term CrM loading did not significantly increase resting ACP measures. However, CrM did affect the ACP responses specific to dehydration, because a small to moderate effect was reported between pre-DHY and post-DHY ACP measures. Our data showed a greater increase in ACP in the CrM trial than in the P trial at the post-DHY time point. It is important to note that the subjects exercised for 2 hours, divided between bicycling and walking, to achieve DHY; however, measures were taken 50 minutes after the activity. We also found that CrM had an effect on the dehydrated subjects exercising in the heat, because a small to moderate interaction was reported between pre-DHY and 1 post-HTT exercise for the trials. In addition, we discovered that CrM supplementation caused a delay in the return of postexercise ACP measures to resting values in these dehydrated subjects exercising in the heat.
As expected, our results are consistent with the findings of previous authors who have noted body mass increases in the first days of CrM supplementation, likely from a decrease in fluid excretion and an increase in fluid retention.2–9,11–13,18 Because of the unyielding dimensions of the anterior compartment, any increase in fluid retention from CrM supplementation that leads to a direct swelling of the muscle fibers in the compartment might overwhelm the limited space available for normal increases in ACP with exercise. This potential mechanism of increased fluid that shifts to within the interstitial space of the anterior compartment could support the significant increase in ACP.
Our findings are somewhat consistent with the 2 recently published studies investigating CrM supplementation and ACP,16,17 although we observed increases in ACP with exercise and a delay in pressure recovery but not increases in resting values. Schroeder et al16 and Potteiger et al17 established a significant pattern of increased resting and postexercise ACP and a delay in recovery from the increased pressure in individuals supplementing with creatine. In conjunction with the significantly increased ACP measurements, both Schroeder et al16 and Potteiger et al17 noted multiple subjects suffering from symptoms consistent with anterior compartment syndrome. Subjective complaints of tightness, aching, cramping, or burning pain over the anterior compartment were reported in the 2 studies.16,17 Other than a case report also published by Potteiger et al,15 no other published study includes symptoms of increased ACP.4,11,13 No evidence was observed in our study that short-term CrM loading led to the signs and symptoms consistent with anterior compartment syndrome in a dehydrated athlete performing intense, intermittent, and prolonged exercise in the heat and humidity. None of the subjects in this study complained of lower extremity aching, cramping, burning pain, or tightness over the area of the anterior compartment of the lower leg, which is consistent with most of the literature.2–7,11,13
However, multiple differences exist between our study and the previous 2 investigations concerned with CrM supplementation and ACP measures.16,17 First, the principal difference critical to this investigation was the dual stresses of dehydration and performing the exercise protocol in the heat. One possible explanation could be that during the dehydrated state, the pool of increased fluid retention resulting from initial CrM supplementation is indeed usable by the body and shifts from within the osteofacial space rather than becoming trapped in the compartment. The pressure may not have increased as dramatically in these dehydrated subjects supplementing with CrM and exercising in the heat because fluid was needed by the body and the water was pulled from the osteofacial space rather than being retained in the closed space. This mechanism may be additionally supported by the finding of increased ACP in the CrM trial and decreased pressures with the P after rehydration of 1 L of water. It is important to consider that the negative effects of dehydration are far more detrimental than any benefit resulting from a diminished ACP.
A second difference between this and previous studies involves the posture of the subjects.16,17,23 In our study, subjects were positioned standing upright with weight on the contralateral leg rather than the traditional supine positioning. However, because of the crossover design, comparisons can still be made among trials and subjects. Finally, exercise protocols in previous investigations were quite different in intensity and duration, whereas we used traditional clinical protocols for assessing ACP measurements.16,17,23 Specifically, previous authors16,17 used treadmill running with no grade at 80% of maximal aerobic power for 20 minutes. This exercise protocol was developed and used by clinicians because symptoms of anterior compartment syndrome could be generally reproduced by symptomatic individuals within the boundaries of the protocol.23 However, we employed a unique protocol combining aerobic and anaerobic exercise to mimic sport participation in a practice or competition. Although accurate comparisons with prior studies may be difficult, our results indicate that the intermittent and prolonged nature of our exercise protocol did not raise ACP as dramatically as did the continuous, 20-minute exercise protocol of the traditional testing procedure. This may be a result of the inability of the pressure to accumulate because of the recovery period during the exercise.
A high dose of CrM supplementation during exercise periods of increased thermal stress showed a mild to moderate trend toward increased ACP measures in dehydrated males. However, our results do not support the American College of Sports Medicine's recommendation, because no associated symptoms of anterior compartment syndrome were seen. The differences were minimal, and the increased pressures readily equalized after intermittent exercise. Future researchers should include a similar dehydration protocol, followed by a longer, continuous bout of exercise. In addition, it would be beneficial to determine whether individuals who suffer from anterior compartment syndrome have increased symptoms during CrM supplementation. Finally, it is important that medical personnel, including certified athletic trainers and team physicians, consider risk factors associated with increasing ACP when treating athletes supplementing with CrM.
Acknowledgments
This research was supported by the National Athletic Trainers' Association Research & Education Foundation (Dallas, TX) and Stryker Instruments (Kalamazoo, MI). We thank the 11 dedicated subjects for their commitment to the study. Also, we gratefully acknowledge the technical assistance provided by Dr Jeff Volek, Michael D'Alfonso, Katie Krog, and Alex Seen.
REFERENCES
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Volek JS. Creatine supplementation: its role in human performance. Clin Sports Med. 1999;18:651–666. doi: 10.1016/s0278-5919(05)70174-5. [DOI] [PubMed] [Google Scholar]
- Juhn MS, Tarnopolsky M. Oral creatine supplementation and athletic performance: a critical review. Clin J Sport Med. 1998;8:286–297. doi: 10.1097/00042752-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Terjung RL, Clarkson P, Eichner ER. American College of Sports Medicine roundtable: the physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. et al. [DOI] [PubMed] [Google Scholar]
- Kreider RB, Ferreira M, Wilson M. Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. et al. [DOI] [PubMed] [Google Scholar]
- Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gomez AL, Kraemer WJ. Physiological responses to short-term exercise in the heat after creatine loading. Med Sci Sports Exerc. 2001;33:1101–1108. doi: 10.1097/00005768-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Peeters BM, Lantz CD, Mayhew JL. Effect of oral creatine monohydrate and creatine phosphate supplementation on maximal strength indices, body composition, and blood pressure. J Strength Cond Res. 1999;13:3–9. [Google Scholar]
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatine, or CK activity in men and women. Med Sci Sports Exerc. 2000;32:291–296. doi: 10.1097/00005768-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Volek JS, Duncan ND, Mazzetti SA. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. et al. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Webster MJ, Erdmann LD, Clark RD. Creatine supplementation: effect on supramaximal exercise performance at two levels of acute hypohydration. J Strength Cond Res. 2000;14:214–219. [Google Scholar]
- Schilling BK, Stone MH, Utter A. Creatine supplementation and health variables: a retrospective study. Med Sci Sports Exerc. 2001;33:183–188. doi: 10.1097/00005768-200102000-00002. et al. [DOI] [PubMed] [Google Scholar]
- Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 1999;31:1108–1110. doi: 10.1097/00005768-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Juhn MS, Tarnopolsky M. Potential side effects of oral creatine supplementation: a critical review. Clin J Sport Med. 1998;8:298–304. doi: 10.1097/00042752-199810000-00007. [DOI] [PubMed] [Google Scholar]
- Robinson SJ. Acute quadriceps compartment syndrome and rhabomyolysis in a weight lifter using high-dose creatine supplementation. J Am Board Fam Pract. 2000;13:134–137. doi: 10.3122/15572625-13-2-134. [DOI] [PubMed] [Google Scholar]
- Potteiger JA, Randall JC, Scroeder C, Magee LM, Hulver MW. Elevated anterior compartment pressure in the leg after creatine supplementation: a controlled case report. J Athl Train. 2001;36:85–88. [PMC free article] [PubMed] [Google Scholar]
- Schroeder C, Potteiger J, Randall J. The effects of creatine dietary supplementation on anterior compartment pressure in the lower leg during rest and following exercise. Clin J Sport Med. 2001;11:87–95. doi: 10.1097/00042752-200104000-00005. et al. [DOI] [PubMed] [Google Scholar]
- Potteiger JA, Carper MJ, Randall JC, Magee LJ, Jacobsen DJ, Hulver MW. Changes in lower leg anterior compartment pressure before, during, and after creatine supplementation. J Athl Train. 2002;37:157–163. [PMC free article] [PubMed] [Google Scholar]
- Kamber M, Koster M, Kreis R, Walker G, Boesch C, Hoppeler H. Creatine supplementation, part I: performance, clinical chemistry, and muscle volume. Med Sci Sports Exerc. 1999;31:1763–1769. doi: 10.1097/00005768-199912000-00011. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJM. Nutritional supplements: effects on exercise performance and metabolism. In: Lamb DR, Murray R, eds. The Metabolic Basis of Performance in Exercise and Sport. Carmel, IN: Cooper Publishing; 1999;209–220.
- Ziegenfuss TN, Lowery LM, Lemon PWR. Acute fluid volume changes in men during three days of creatine supplementation. J Exerc Physiol. 1998;1:1–7. [Google Scholar]
- Wallensten R, Eklund B. Intramuscular pressures and muscle metabolism after short-term and long-term exercise. Int J Sports Med. 1983;4:231–235. doi: 10.1055/s-2008-1026040. [DOI] [PubMed] [Google Scholar]
- Casa DJ. Exercise in the heat, I: fundamentals of thermal physiology, performance implications, and dehydration. J Athl Train. 1999;34:246–252. [PMC free article] [PubMed] [Google Scholar]
- Pedowitz RA, Hargens AR, Mubarak SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35–40. doi: 10.1177/036354659001800106. [DOI] [PubMed] [Google Scholar]