Abstract
Rats acquired aversions to food pellets when a previously-trained signal for that food was paired with a toxin, but only after minimal signal-food training. After extended signal-food training, signal-toxin pairings had no effect on food consumption, even after manipulations that enhanced the associability of the signal. By contrast, conditioned responding to the signal retained its sensitivity to devaluation of the food reinforcer by food-toxin pairings after extended training. These results suggest that the nature of associatively-activated event representations changes over the course of training.
Within most modern descriptions of Pavlovian conditioning, pairings of a conditioned stimulus (CS) and an unconditioned stimulus (US) endow the CS with the ability to activate a memorial representation of the US. One consequence of this activation is that the CS often comes to elicit a conditioned response (CR) that shares features with the unconditioned response. Considerable evidence indicates that CS-activation of US representations may serve a number of functions beyond determination of CR form, influencing performance in a variety of learning tasks, including reinforcer revaluation (e.g., Balleine & Dickinson, 1998; Holland & Rescorla, 1975), mediated learning (Dwyer, 2003; Hall, 1996; Holland, 1981, 1990), differential outcome expectancy (e.g., Blundell, Hall, & Killcross, 2001; Rescorla, 1992; Trapold & Overmier, 1972), and acquired equivalence (Hall, 1996).
Hall (1996) and Holland (1990) described a number of contexts in which associatively-activated event representations appear to substitute for their referents in the control of learning and action. Perhaps the most commonly investigated instance is that of reinforcer revaluation, in which the reinforcement value of the US is changed after the completion of CS-US training. For example, Holland and Rescorla (1975) and Holland and Straub (1979) first presented rats with pairings of a tone CS with a food pellet US, and then paired access to those pellets in the home cage with the induction of illness. Subsequent tests with the tone alone showed significant reductions in conditioned responses (CRs) relative to the performance of rats that received food and illness unpaired. In experiments such as these, performance of CRs is thought to be mediated by a CS-activated representation of the US, which substitutes for a representation activated by the US itself in the control of behavior. Post-training changes in the value of the US representation then could result in spontaneous changes in CRs elicited by the CS.
Hall (1996) and Holland (1981, 1990) showed that associatively-activated US representations may also substitute for their referents in the acquisition of new learning. For example, in several experiments (Holland, 1981, 1990), which used a variety of auditory or visual CSs and solid or liquid food USs, rats first received CS-food pairings. Then, in the absence of the food US, the CS was either paired with toxin-induced illness, or presented explicitly unpaired with that illness. Finally, consumption of the food was assessed in the absence of the CS. Rats that received CS-illness pairings consumed less food than rats that received CS and illness unpaired. Holland (1981) claimed that the learned food aversion was mediated by a CS-activated representation of the food: pairing of that representation of the food with illness was sufficient to establish an aversion to the food itself.
Later experiments (Holland, 1990, Exp. 10; 1998, Exps. 1, 2) showed a limitation on the conditions under which this mediated food aversion learning occurred, namely the number of CS-food pairings in the initial training phase. In those experiments, mediated food aversion learning was observed only after relatively small numbers (16–20) of CS-food pairings; after as few as 40 pairings there was no evidence for representation-mediated food aversion learning. A simple account for this outcome is that CSs lose the ability to activate a US representation as conditioning proceeds, and CRs are instead increasingly elicited by stimulus-response habits (e.g., Adams & Dickinson, 1981; Bussey, Muir, Everitt, & Robbins, 1996; Kimble & Perlmuter, 1970; Poldrack & Packard, 2003). However, comparable studies of the effects of amount of training on sensitivity of Pavlovian CRs to reinforcer revaluation procedures (Holland, 1998, Exp. 3) showed that representation-mediated performance of CRs was unaffected by the amount of initial CS-food training in this conditioning procedure; responding to the CS was equally reduced by post-training food-toxin pairings after 16 or 160 CS-food pairings. Thus, although extended CS-food training reduced rats’ use of an associatively-activated representation of food to establish new learning about that food, it had no effect on the mediation of CR performance by such a representation.
To accommodate this dissociation between the effects of amount of training on mediated learning and reinforcer devaluation, Holland (1998) proposed that the nature of associatively-activated event representations changes over the course of learning. Although the notion of an associatively-activated US representation is frequently invoked in theoretical descriptions of conditioning, the nature of such representations is seldom specified. Holland (1990) suggested that an associatively-activated event representation refers simply to a set of nervous system processes normally evoked by the US, which come under control of a CS through learning. Early in training, CSs may access a different subset of these processes than they do later in training. For example, Holland (1998) suggested that early in training CSs may activate perceptual processing normally activated only by the US, but are unable to do so later in training. Casually speaking, early in training, rats may functionally taste the food in the presence of the CS prior to food delivery itself. Thus, when a minimally-trained CS for food is paired with toxin, perceptual processing of the food flavor, like that produced by presentation of the food itself, is paired with illness, establishing a flavor-illness association. By this view, the CS alone loses its ability to activate perceptual processing of the upcoming food as conditioning proceeds, and so CS-illness pairings would not provide the taste-illness pairings required for taste aversion learning. By contrast, the sensitivity of previously-established CRs to reinforcer revaluation may be mediated by less transient aspects of an associatively-activated US representation, which incorporate the US’s incentive value but do not provoke perceptual processing of that event in its absence.
However, a much simpler account for both the loss of mediated aversion learning and the maintenance of reinforcer devaluation effects with extended training in Holland’s (1998) experiments may be provided by extension of a model of conditioning proposed by Pearce and Hall (1980). Within that model, the learning rate parameter (∀, termed “associability”) of a CS declines as its consequences become more reliably predicted. With simple pairings of a single CS with a US, as that CS becomes a better predictor of the US, its associability declines. Thus, the more CS-US pairings, the less that CS will be able to acquire new learning. Indeed, within this model, a major determinant of negatively-accelerated learning curves is the gradual loss of a CS’s associability as it becomes a more reliable predictor of the US. Notably, in this model, CS associability is assumed to influence only learning, and not performance; the ability of a CS to elicit CRs, once they are established, is not influenced by its associability. Thus, CSs maintain their ability to elicit CRs even as they lose the ability to acquire new learning.
Applying this model to Holland’s (1998) experiments, it could be argued that extended training of a CS not only reduces the ability of that CS itself to enter into new associations, but also the ability of any representation that the CS activates. Thus, with extended training of a CS, a CS-activated representation of food would lose its ability to enter into new associations with illness. At the same time, because the associability of a CS does not affect its previously-established ability to activate a US representation, extended training would not affect sensitivity of CRs to reinforcer devaluation procedures.
The experiments described in this article were designed to evaluate a Pearce-Hall (1980)-based account for Holland’s (1998) observations that mediated food aversion learning was obtained after small numbers of CS-food pairings but not after extensive CS-food training. They replicated the amount of training effect observed by Holland (1998) and examined the effects of two manipulations designed to restore or maintain the associability of extensively-trained CSs. In Experiments 1 and 2, after extensive CS-US pairings, CS associability was restored by a brief extinction phase prior to assessment of new learning, and in Experiment 3, CS associability was maintained over extensive training by intermixing nonreinforced CS presentations throughout training. In both cases, violation of CS-US expectancy produced by US omission should restore or maintain CS associability, relative to treatments without expectancy-violating extinction trials. In turn, this restoration of associability should enable the acquisition of a mediated flavor aversion, even after extended CS-US training.
Experiment 1
According to the Pearce-Hall model, extensive CS-US pairings reduce the associability of the CS, making it less sensitive to new learning. Hall and Pearce (1979) found that interposing an extinction (CS-alone) trial between extended tone-shock training and pairings of that tone with a more intense shock enhanced learning of associations between that tone and the more intense shock. Within the Pearce-Hall model, by generating a discrepancy between the expected and actual reinforcer received (“surprise”), shock omission on the extinction trial enhanced the associability of the tone on subsequent trials, allowing it to more rapidly accrue associative strength based on the new, more intense shock. At the same time, because the tone’s associability would be low on the extinction trial itself, shock omission would have little direct extinction effect on the tone’s existing associative strength.
In Experiment 1, rats first received extended training with one CS and minimal training with a second CS; both CSs were paired with the same food pellet reinforcer. Then, separate groups of rats received a brief extinction or “surprise” phase with the minimally trained CS, the extensively trained CS, or no explicit event. Next, one of the CSs was paired with LiCl injection in an attempt to establish an aversion to the food pellets, mediated by a CS-activated representation of food. Finally, pellet consumption was examined to assess aversion learning. If representation-mediated learning of a food aversion is reduced after extended CS-US pairings because the associability of the CS-activated representation of food pellets is driven down (as suggested by the Pearce-Hall model), then the surprise occasioned by a brief period of nonreinforcement of that CS should enhance its associability. Thus, rats that receive brief extinction of the extensively-trained CS before that CS is paired with LiCl pairings should show more evidence of food pellet aversion than rats that do not receive extinction trials. By contrast, the minimally-trained CS is unlikely to have lost much of its associability (e.g., Holland, Bashaw, & Quinn, 2002; Kaye & Pearce, 1984), and so the surprise trials are unlikely to have much incremental effect on that CS’s associability. Indeed, that treatment might reduce the minimally-trained CS’s ability to activate a representation of the food US, and hence might reduce the formation of a mediated food aversion relative to rats that do not receive non reinforced presentations of that CS.
Methods
Subjects and apparatus
Thirty male albino rats (CD strain, Charles River Laboratories, Raleigh, North Carolina), which weighed 300–325 g upon arrival to the vivarium, were housed individually with ad lib access to water. The rats were maintained at 85% of their baseline weights by limiting their access to food to a single daily meal. The vivarium was climate controlled and illuminated from 6 am to 8 pm.
The apparatus consisted of eight individual chambers (22.9 X 20.3 X 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. A dimly illuminated food cup was recessed in the center of one end wall. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry. Each chamber was enclosed in a sound-resistant shell. A 6-w house light was mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food cup. Ventilation fans provided masking noise (70 dB). Constant dim illumination was provided by a 6-w lamp behind a dense red lens mounted on the ceiling of the shell.
Procedures
The top of Table 1 shows an outline of the training procedures that were used in Experiment 1. The rats were first trained to eat from the recessed food cup in a single 64-min session, which included 16 deliveries of the reinforcer, two 45-mg Noyes food pellets (Research Diets, New Brunswick, NJ). Next, the rats received 8 64-min training sessions to establish minimal training of one CS and extended training of another CS. In each of these sessions, there were 14 reinforced presentations of one CS and 2 reinforced presentations of the other. For half of the rats, the minimally-trained CS was a 10-s illumination of the light and the extensively-trained CS was a 10-s 78-db 1,500-Hz tone. For the other half of the rats, the identities of the two CSs were reversed. Then, the rats were divided into four groups (ns = 7 or 8) and given a single 16-min session designed to increase the associability of the extensively-trained CS in Group Extended-Surprise prior to mediated aversion training. In this session, the rats in Groups Extended-Surprise and Minimal-Surprise received four nonreinforced presentations of the extensively-trained CS or the minimally-trained CS, respectively, and the rats in Groups Extended-No Surprise and Minimal-No Surprise received only placement in the chambers.
Table 1.
Outline of Procedures of Experiments 1 and 2
| Experiment 1 | |||||
|---|---|---|---|---|---|
| Group Name | Training | Surprise | Pretest | Mediated Aversion | Test |
| Extended-Surprise | 112 CSextended → food
16 CSminimal →food |
CSextended → nothing | food? | CSextended → LiCl | food? |
| Extended-No Surprise | 112 CSextended → food
16 CSminimal → food |
chamber only | food? | CSextended → LiCl | food? |
| Minimal-Surprise | 112 CSextended → food
16 CSminimal → food |
CSminimal → nothing | food? | CSminimal → LiCl | food? |
| Minimal-No Surprise | 112CSextended → food
16 CSminimal → food |
chamber only | food? | CSminimal → LiCl | food? |
| Experiment 2 | |||||
| Group Name | Training | Surprise | Test | ||
| Surprise | 112 CSextended → food
16 CSminimal → food |
CSextended → nothing | CSextended → new food | ||
| No Surprise | 112 CSExtended → food
16 CSminimal → food |
chamber only | CSextended → new food | ||
Notes.The numbers listed in the training column refer to the total number of trials of each type. In Experiment 2, the new food was delivered in the original food cup in half of the rats in each group and in a new food cup in the other half of the rats. CS = conditioned stimulus, LiCl = lithium chloride, → followed by.
On the next day, the rats received 10 min exposure in the experimental chambers to a white ceramic bowl that contained 100 pellets, as a pretest of the consumption test procedure. Over the next four days, the rats received 4 5-min sessions designed to establish an aversion to the food pellet reinforcer, by pairing a presentation of one of the CSs (but not pellets) with the toxin lithium chloride (LiCl). In the first and third of these sessions, there was a single 10-s presentation of one of the CSs, in the absence of food. Immediately after this CS presentation, the rats were removed from the chambers and injected with 5 ml/kg 0.3-M LiCl and returned to their home cages. For the rats in Extended-Surprise and Extended-No Surprise, this CS was the extensively-trained CS and for the remaining rats it was the minimally-trained CS. The identity (light or tone) of the CS, whether the surprise session included the extensively-trained CS or not, and whether the toxin-paired CS was the minimally-trained or the extensively-trained CS, were completely counterbalanced. In the second and fourth sessions of the mediated aversion training phase, the rats were placed in the chambers for 5 min but no events were delivered. Finally, on the next day, the rats received 10 min exposure in the experimental chambers to a white ceramic bowl that contained 100 pellets, as a test of the effects of the mediated aversion training sessions.
Results
Figure 1 shows the acquisition of conditioned responding during the initial training phase. Conditioned food cup responding was acquired rapidly to each CS, although the tone elicited more responding than the light, especially with minimal training. As would be anticipated, responding was greater to the extensively-trained CSs than to the minimally trained CSs. Food cup responding was subjected to a 5-way ANOVA, with variables of cue identity (tone or light), subsequent mediated aversion condition assignment (whether the minimally- or extensively-trained CS would be paired with LiCl), subsequent surprise assignment (surprise/extinction or no surprise), amount of training of the CS (miminal or extended), and sessions. The effects of cue identity, F(1, 22) = 8.00, number of training trials, F(1, 22) = 126.11, and sessions, F(7, 154) = 42.53, were significant, as were the interactions of cue identity with number of training trials, F(1, 22) = 109.61, and sessions, F(7, 154) = 2.86, and the interaction of the number of training trials with sessions, F(7, 154) = 4.10. Neither of the subsequent assignment variables nor any of their interactions was significant, Fs < 1.5. A similar ANOVA of baseline responding prior to each trial (means < 10%) showed no significant effects or interactions, Fs < 1.5.
Figure 1.
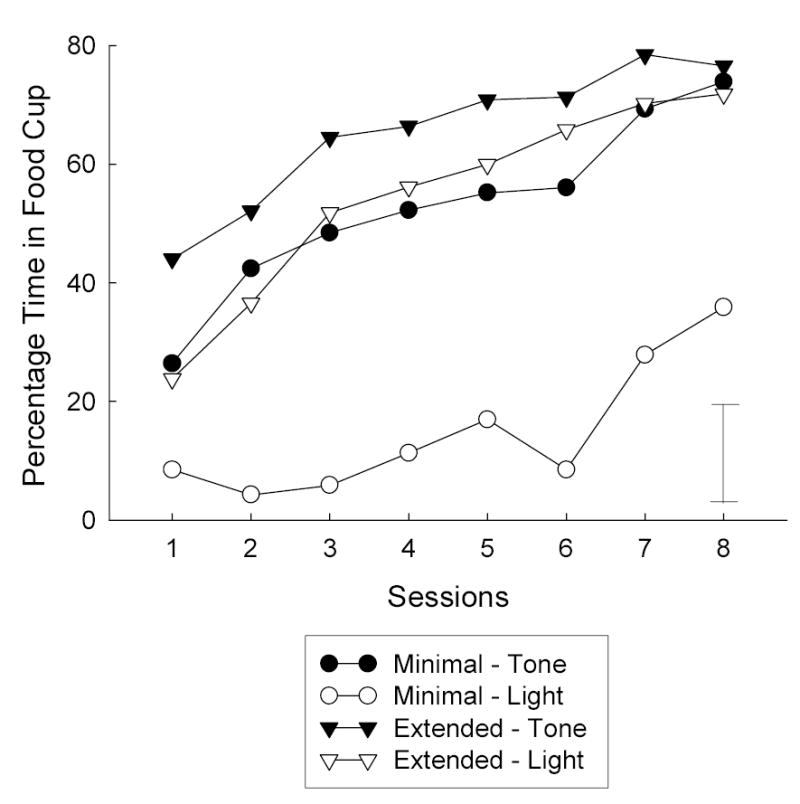
Acquisition of food cup responding in the initial training phase of Experiment 1. In each session there were two presentations of the conditioned stimulus that received minimal training and 14 of the conditioned stimulus that received extended training. The error bar indicates two times the within-subjects mean standard error.
Table 2 shows responding to the CSs in the surprise and mediated learning sessions. Responding in theses sessions maintained the patterns seen at the end of initial training. The surprise treatment partially extinguished responding: the rats that received the surprise treatment showed less responding in the mediated aversion sessions than the rats that received only context exposure in those sessions. A cue identity X amount of training ANOVA showed significant main effects of both variables, Fs (1, 11) > 7.66, but their interaction was not reliable, F<1. A cue identity X previous surprise treatment X amount of training of the surprise cue X session ANOVA of responding on the mediated aversion trials showed significant main effects of cue identity, previous surprise treatment, and amount of training, Fs(1, 22) > 4.11, but not of sessions, F(1, 22) = 3.12. In addition, amount of training interacted significantly with both cue identity and prior surprise treatment, Fs(1,22) > 4.82; cue identity mattered more after few training trials, and the surprise trials produced greater loss after extended training. ANOVA of pre-CS responding showed no effects, Fs < 1.
Table 2.
Responding in the Surprise and Mediated Aversion Phases of Experiment 1
| Group | Surprise | Mediated Aversion | |
|---|---|---|---|
| Extended-Surprise | trials 1-4 | trial 1 | trial 2 |
| Tone | 65.2 ± 1.3 | 33.2 ± 14.0 | 10.1 ± 11.6 |
| Light | 48.7 ± 5.4 | 31.9 ± 5.2 | 25.2 ± 14.5 |
| Pre-CS | 4.8 ± 3.2 | 4.1 ± 1.9 | 3.8 ± 3.1 |
| Extended-No Surprise | |||
| Tone | ------ | 73.4 ± 14.0 | 60.9 ± 11.6 |
| Light | ------ | 68.9 ± 6.7 | 39.6 ± 15.4 |
| Pre-CS | ------ | 5.0 ± 2.3 | 4.0 ± 2.1 |
| Minimal-Surprise | |||
| Tone | 45.7 ± 16.8 | 21.1 ± 15.4 | 20.6 ± 14.7 |
| Light | 17.0 ± 12.0 | 2.7 ± 2.1 | 1.6 ± 1.8 |
| Pre-CS | 3.5 ± 1.6 | 4.1 ± 2.0 | 2.8 ± 1.9 |
| Minimal-No Surprise | |||
| Tone | ------ | 47.5 ± 14.2 | 31.1 ± 20.5 |
| Light | ------ | 15.3 ± 12.0 | 18.2 ± 8.8 |
| Pre-CS | ------ | 3.9 ± 1.9 | 2.9 ± 1.6 |
Notes. Entries are mean (±sem) percentage time in food cup.
Figure 2 shows the primary data from this experiment, the consumption of food pellets from the ceramic bowls in the experimental chambers. The rats that received pairings of the minimally-trained CS with toxin consumed fewer pellets than rats that received the extensively-trained CS paired with toxin. This outcome is consistent with the claim that the rats acquired a stronger aversion to the food when a minimally-trained CS was paired with toxin than when an extensively-trained CS was paired with toxin, as noted by Holland (1998).
Figure 2.
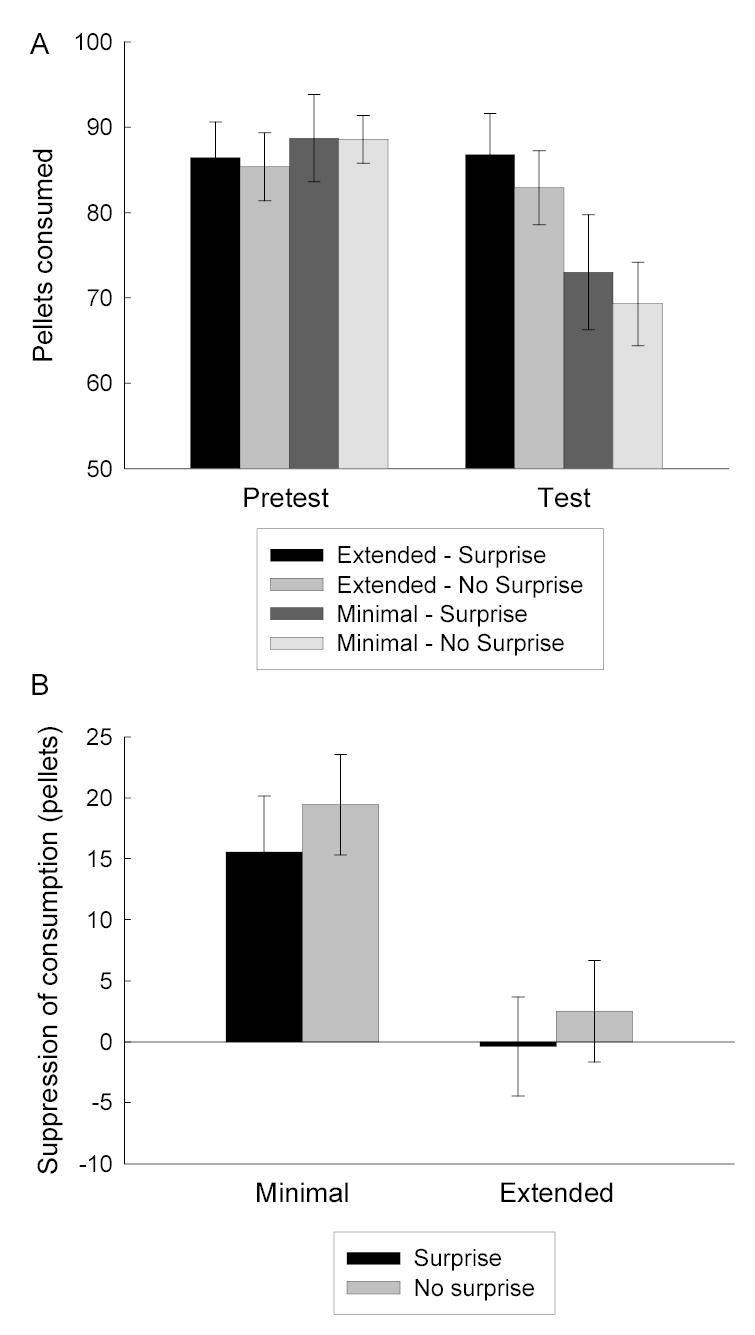
Mediated food aversion learning in Experiment 1. Panel A shows mean (±sem) food consumption in the pretest and test phases and Panel B shows the mean (±sem) change in consumption between those tests (pretest minus test). In Panel B, reductions in consumption (positive change scores) reflect the acquisition of a food aversion.
Critically, the surprise manipulation had no effect on mediated aversion learning. Rats that received toxin after the minimally-trained CS showed less consumption in the test session (right bars in Figure 2A) and more reduction of consumption (Figure 2B) from the pretest levels than the rats that were made ill after the extensively-trained CS. There were no differences among the groups in consumption during the pretest (left bars in Figure 2A).
A cue identity X surprise X amount of training of the toxin-paired CS ANOVA of pretest consumption showed no significant effects (Fs < 1, except for the cue identity X amount of training interaction, F(1,22) = 2.89, p > .10). A comparable ANOVA of test responding showed a reliable effect of amount of training of the toxin-paired CS, F(1, 22) = 8.10; remaining Fs < 1, except for the cue identity X amount of training interaction, F(1,22) = 3.30, p = .08. Finally, a comparable ANOVA that also included test vs pretest as a variable showed a significant effect of that variable, F(1, 22) = 20.72, and its interaction with the amount of training of the toxin-paired CS, F(1, 22) = 16.17. No other effect or interaction approached significance except the cue identity X amount of training interaction, F(1,22) = 3.93, p = .06.
Discussion
The results of Experiment 1 replicated those of Holland (1998): food consumption was reduced after pairings of a minimally-trained CS with LiCl but not after pairings of an extensively-trained CS with LiCl. Although unlike in those previous experiments, Experiment 1 did not include nonassociative controls, e.g., toxin presentations unpaired with any CS (a control provided in Experiment 3), it seems reasonable to infer from these data that acquisition of a food aversion, mediated by a CS-activated representation of food, was greater after minimal than after extensive training. Notably, CS extinction trials administered prior to CS-LiCl pairings, intended to restore the associability of the food representation activated by the extensively-trained CS, were not effective in altering the acquisition of the mediated taste aversion after either minimal or extended training. Thus, there was no evidence that losses in the associability of a CS-activated representation of food pellets with extended CS-US training were critically involved in the loss of susceptibility to mediated aversion learning.
Regardless of the role of associability changes, the observation of differential effects of CS-toxin pairings on mediated aversion learning after minimal and extensive CS-food training in this procedure demands that the food representations activated by the two CSs were distinguished. Each rat received both extensive training with one CS and minimal training with another CS, each paired with the same food reinforcer. If both CSs activated the same food representation, then it should not have mattered which CS was paired with toxin. But a food aversion was formed only if the minimally-trained CS was paired with toxin.
Finally, it is notable that the brief extinction phase (4 trials) substantially reduced CRs to the extinguished CSs (Table 2). In the case of the minimally-trained CS, this observation indicates that even proportionally large losses in CRs to the CS had no observable effect on that CS’s ability to activate a representation of food pellets for mediated learning of a taste aversion. This effect is reminiscent of Rescorla’s observations that Pavlovian CSs apparently still controlled specific outcome expectancies after extensive extinction of CRs and even counterconditioning with new USs (Rescorla, 1996a, b). Furthermore, this observation reduces concerns that with the extensively-trained CS, detrimental effects of extinction on the ability of the CS to activate a food representation may have offset any advantageous effects of the surprise treatment on the associability of the CS-activated food representation.
Experiment 2
The logic of Experiment 1 demands that the surprise procedure used indeed restores the associability of the extensively-trained CS. The absence of any enhancement of mediated aversion learning by surprise in Experiment 1 may simply reflect ineffectiveness of that procedure in restoring associability, rather than a lack of an effect of such a restoration. Experiment 2 considered whether the surprise procedures of Experiment 1 are sufficient to restore CS associability, as measured by simple acquisition of new learning to the extensively-trained CS itself. The acquisition and surprise procedures were identical to those used in Experiment 1. However, the mediated aversion phase was replaced by pairings of the test CS with a new, higher-magnitude (5 pellets rather than 2) and qualitatively-preferred (fruit-punch flavored sucrose vs. grain) reinforcer. If the surprise procedure of Experiment 1 restored putative losses in the associability of the extensively-trained CS, then rats that received that treatment should show more rapid acquisition of conditioned responding appropriate to the new reinforcer. For half of the rats, the new reinforcer was delivered to the same food cup as the original reinforcer, and for half it was delivered to a previously-unused cup on the opposite side of the chamber. The purpose of using two different delivery sites for some of the rats in the test phase was to evaluate any role of summation of, or competition between, the peripheral CRs established with the old and new reinforcers. Because the primary concern of the present studies is why extended training reduces mediated aversion learning, in Experiment 2 only the extensively-trained CS served as a target cue.
Subjects and apparatus
The subjects were 24 male albino rats obtained and maintained as in Experiment 1. The apparatus included 6 of the chambers used in Experiment 1, except a second recessed food cup, on the opposite wall of the chamber, was made available in the test phase for half of the rats.
Procedures
The lower portion of Table 1 shows an outline of the procedures of Experiment 2. The training and surprise sessions were identical to those received by the rats in the Extended-Surprise and Extended-No Surprise groups of Experiment 1. However, instead of mediated aversion training, these rats received two 64-min sessions, each with 8 pairings of the extensively-trained CS with a new reinforcer, 5 fruit-punch flavored sucrose pellets (Research Diets, Inc.) to assess the ability of the surprise session to enhance the associability of that CS. For half the rats in each training and counterbalancing condition, the new reinforcer was delivered to the same food cup as the other pellets, but for the other half of the rats, it was delivered to a similar food cup in the opposite wall of the chamber. For these latter rats, the original food cup opening was blocked in this test.
Results
As in Experiment 1, conditioned food cup responding was acquired rapidly to each CS, although the tone elicited more responding than the light early in training. On the final training session, the percentages of time in the food cup during the extensively-trained light and tone stimuli were 77.1 ± 3.8% and 76.4 ± 4.1%, respectively, and to the minimally-trained light and tone, they were 42.1 ± 6.5% and 77.4 ± 5.1%. Pre-CS responding was less than 10%, and did not differ across groups, Fs < 1. Food cup responding during the CSs was subjected to a 3-way ANOVA, with variables of cue identity (tone or light), subsequent surprise assignment (surprise or no surprise), and amount of training of the CS (minimal or extensive). The main effects of cue identity, F(1, 20) = 8.27, and amount of training, F(1, 20) = 34.92, and the interaction of cue identity with amount of training, F(1, 20) = 36.06, were all significant; the effects of subsequent assignment to surprise condition was not, F < 1.
As in Experiment 1, responding in the surprise session maintained the pattern seen at the end of initial training. Responding to the extensively trained light and tone was 48.0 ± 7.0% and 53.0 ± 6.9%, respectively, which did not differ significantly, F < 1, and pre-CS responding was 3.6 ± 3.1%.
Figure 3 shows the primary data of Experiment 2, the acquisition of CRs to the extensively-trained CS as it was paired with a new reinforcer. In all conditions, responding rapidly rose to a new higher asymptote. However, in the first test session, the rats that had received nonreinforced surprise presentations of the extensively-trained CS prior to test pairings with the new US showed more responding than the rats that did not receive those presentations, despite starting that session (trial 1) with lower levels of responding to the CS. This superiority in acquisition was observed regardless of whether the new reinforcer was delivered to the original food cup location or a new location.
Figure 3.
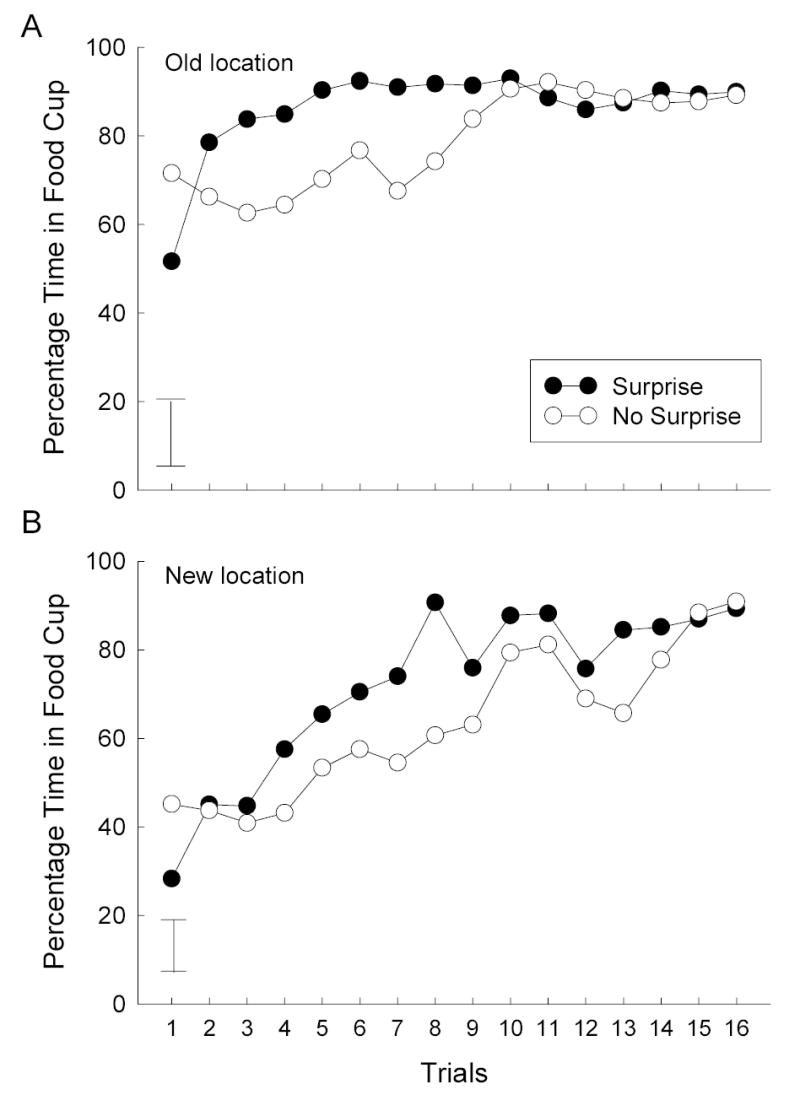
Acquisition of new learning in the test phase of Experiment 2. The error bars show two times the between-groups mean standard error.
Separate CS identity X surprise condition X trials ANOVAs for responding in the first test session were conducted for the rats that received the new reinforcer in the old and new locations. For both locations, there were significant main effects of surprise, Fs(1, 8) > 6.16, and trials, Fs(7, 56) > 13.13, and significant surprise X trials interactions, Fs(7, 56) > 11.94. Separate analyses for the first trial only also showed significant main effects of surprise, Fs(1, 8) > 5.29; note that the differences on trial one were in the opposite direction as those in the remaining sessions. This difference in responding on trial 1 reflects extinction of responding in the rats that received surprise (extinction) trials with the CS prior to testing.
Pre-CS responding was below 10% in all conditions and did not differ across groups, Fs < 1.
Discussion
The results of Experiment 2 extend the findings of Hall and Pearce (1979) to an appetitive conditioning preparation. Although the brief extinction treatment used to induce surprise produced significant losses in the ability of CSs to elicit already-established CRs, it enhanced the rate of new learning about those CSs. That enhancement occurred regardless of whether the indicator of new learning was the same or different from the CR previously controlled by the CS (entry into the same or different food cup).
These results provide evidence that the extended training used in Experiments 1 and 2 indeed reduced CS associability, and that the surprise procedures used in these experiments are effective in restoring the associability of the extensively-trained CS. If the failure of CS-toxin pairings after extensive CS training to produce a mediated food aversion in Experiment 1 was due to losses in CS associability produced by that extensive training, then the surprise manipulation in that experiment should have restored CS associability and enabled mediated food aversion learning. The lack of such an effect of surprise on mediated aversion learning in Experiment 1 suggests that the inability of the extensively trained CS to establish a mediated taste aversion in Experiment 1 was not due to losses in its associability.
Experiment 3
Within the Pearce-Hall model, CS associability on any trial is determined by the accuracy with which its consequences were predicted on previous trials. Thus, the associability of a CS should be maintained at a higher value with partial reinforcement procedures than with consistent reinforcement procedures. Using orienting responses as an indicator of associability, Pearce and colleagues (e.g., Collins et al., 1983; Collins & Pearce, 1985; Pearce, Kaye, & Collins, 1985; Kaye and Pearce, 1984) found substantially greater associability of CS trained with a variety of partial reinforcement procedures than of CS trained with consistent reinforcement procedures.
In Experiment 3, the acquisition of mediated a taste aversion after CS-illness pairings was examined after different amounts of training of CSs, with either partial or consistent reinforcement procedures. In contrast to Experiment 1, in Experiment 3, the effects of amount of training were evaluated between groups. Each rat received training with two CSs, one consistently paired with food and the other paired with food on 50% of its presentations. Different groups of rats received 20, 40 or 80 training trials. If the amount of training effect is due to associability losses over extended training, and partial reinforcement better maintains CS associability than consistent reinforcement, then with more training trials, the rats that receive pairings of the partially-reinforced CS with toxin should acquire stronger mediated food aversions than the rats that receive pairings of the consistently-reinforced CS with toxin. Although some investigators have reported difficulty in observing another consequence of partial reinforcement, the partial reinforcement extinction effect, in within-subject procedures like these (e.g., Pearce, Redhead, & Aydin, 1997; but see Rescorla, 1999), it is notable that several of Pearce’s demonstrations of greater orienting to partially-reinforced CSs than to consistently-reinforced CSs involved within-subject comparisons.
After assessment of the mediated taste aversion, the same rats were then tested in a reinforcer devaluation procedure, in which the food itself was paired (in some rats) with toxin, and then responding to the CSs was assessed. This test permitted the comparison, in the same rats, of the effects of amount of training and partial reinforcement on performance on the devaluation task with those effects on mediated aversion learning. Recall that Holland (1998, Exp. 3) found that devaluation task performance was insensitive to variations in the amount of training that had critical effects on the observation of mediated aversion learning.
Subjects and apparatus
The subjects were 60 male albino rats obtained and maintained as in Experiments 1 and 2. The apparatus was the same as that used in Experiment 1.
Procedures
Table 3 shows an outline of the procedures of Experiment 3, which was conducted in two similar replications, one with 28 rats and one with 32 rats. All rats first received a food-cup training session, as in Experiments 1 and 2. Then, they were separated into 3 groups, which received different numbers of consistent and partial reinforcement training. The rats in Group 2040 received twenty reinforced presentations of one (CRF) CS and 20 reinforced and 20 nonreinforced presentations of another (PRF) CS. The rats in Group 4080 received 40 reinforced presentations of the CRF CS and 40 reinforced and 40 nonreinforced presentations of the PRF CS. The rats in Group 8080 received 80 reinforced presentations of the CRF CS and 40 reinforced and 40 nonreinforced presentations of the PRF CS. Therefore, in Group 8080 the number of PRF CS and CRF CS presentations (80) was equated and within Groups 2040 and 4080 the number of reinforced presentations was equated (20 and 40, respectively); likewise, the number of CRF CS presentations in Group 4080 and the number of PRF CS presentations in Group 2040 (40) was equated. Each training session included four reinforced and four nonreinforced PRF CS presentations and either 4 (Groups 2040 and 4080) or 8 (Group 8080) reinforced CRF CS presentations. The ITIs (mean = 4 min, range of 2–6 min) were equated across all groups. Thus, the rats in Group 8080 received 10 64-min training sessions, the rats in Group 4040 received 10 48-min training sessions, and the rats in Group 2040 received 5 48-min training sessions. In each group, the identities of the CRF and PRF CSs were counterbalanced (the 10-s light and tone CSs used in the previous studies.)
Table 3.
Outline of Procedures of Experiment 3
| Group | Training | Pretest | Mediated Aversion | Test | Devaluation | Devaluation Test |
|---|---|---|---|---|---|---|
| 2040 | 20 CSCRF → food, 20 CSPRF → food, 20 CSPRF → nothing | Food | CSCRF → LiCl or CSPRF → LiCl or No CS, LiCl | food | food → LiCl or food/LiCl | CSCRF → nothing, CSPRF → nothing |
| 4080 | 40 CSCRF → food, 40 CSPRF → food, 40 CSPRF → nothing | Food | CSCRF → LiCl or CSPRF → LiCl or No CS, LiCl | food | food → LiCl or food/LiCl | CSCRF → nothing, CSPRF → nothing |
| 8080 | 80 CSCRF → food, 40 CSPRF → food, 40 CSPRF → nothing | Food | CSCRF → LiCl or CSPRF → LiCl or No CS, LiCl | food | food → LiCl or food/LiCl | CSCRF → nothing, CSPRF → nothing |
Notes. In the mediated aversion phase, separate groups of rats received injections of LiCl after either CSCRF, CSPRF, or no CS in the experimental chambers. In the devaluation phase, separate groups of rats received food and LiCl injections either paired or unpaired; assignment to paired or unpaired conditions was counterbalanced across all previous treatments. The numbers in the training column refer to the number of trials; CS = conditioned stimulus, CRF = consistent reinforcement, PRF = partial reinforcement, LiCl = lithium chloride.
After a 10-min pretest of consumption of food pellets in the experimental chamber, as in Experiment 1, the rats received mediated aversion training identical to that of Experiment 1, except that the rats rested in their home cages on the second and fourth days, rather than in the experimental chambers. For 6–8 of the rats in each group, the CRF CS was paired with LiCl, for 6–8 rats the PRF CS was paired with LiCl, and for 6 rats no CSs were presented prior to LiCl injections. The identities of these CSs (light or tone) were counterbalanced as closely as possible. On the next day, a consumption test, identical to the pretest session, was conducted.
Finally, a standard reinforcer devaluation procedure was administered over 5 days. On days 1 and 3, half of the rats (counterbalanced across all previous training and testing conditions as much as possible) received 10-min access to the ceramic bowls with 100 pellets in their home cages followed immediately by LiCl injection (Paired condition) and the other half (Unpaired condition) received only the LiCl injections. On days 2 and 4, the rats in the Unpaired condition received 10 min access to the bowls of pellets, but no injections. On the last day of the experiment, all rats received a 32-min test session to assess the effects of flavor aversion training on food cup responding to the two CSs. This session included 4 nonreinforced presentations of each of the two CSs, randomly intermixed.
Results
The rats in all groups rapidly acquired CRs to both CSs in the initial training phase. As in Experiments 1 and 2, the tone elicited more responding than the light, especially with small amounts of training. Partially and consistently reinforced CSs controlled similar levels of conditioned responding; notably, except in Group 8080, the number of reinforced trials for the PRF and CRF CSs were matched. Figure 4 shows responding to the CSs on the final conditioning session. A group (2040, 4080, or 8080) X cue identity (tone or light) X contingency (CRF or PRF) ANOVA showed significant effects of group, F(2, 54) = 5.66, and cue identity, F(1, 54) = 69.31, and a significant interaction of group with cue identity, F(2, 54) = 7.32. With the light CS there was a significant effect of group for both CRF and PRF cues, Fs (1, 54) > 10.49, but with the tone CS those effects were not significant, Fs < 2.11. Pre-CS responding in the final session was 5.1 ± 2.1%, 5.3 ± 2.9%, and 4.9 ± 2.0% in Groups 2040, 4080, and 8080, respectively, F < 1.
Figure 4.
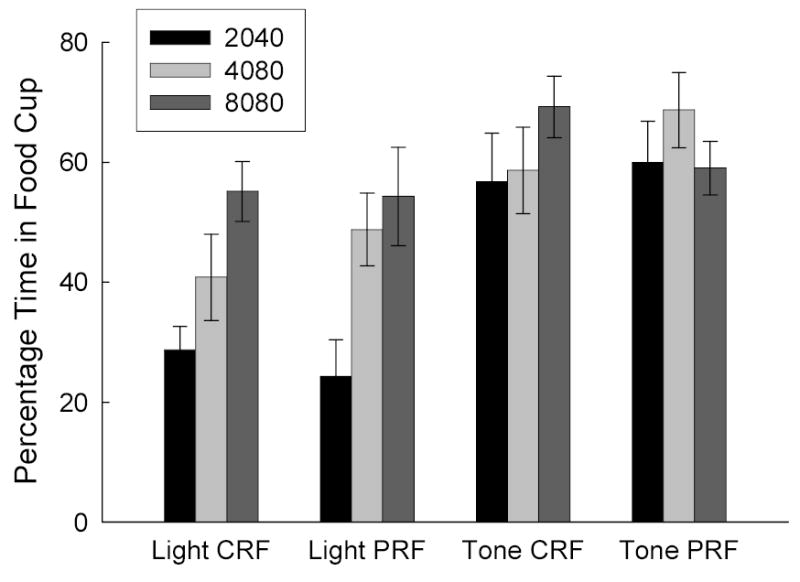
Mean (±sem) food cup responding during the tone and light conditioned stimuli in the last session of initial training in Experiment 3. The first two digits of each group name indicate the total number of CRF trials, and the second two digits indicate the total number of PRF trials received in that phase. CRF = consistently reinforced conditioned stimulus; PRF = partially reinforced conditioned stimulus.
Figure 5 shows the primary data of Experiment 3, the consumption of food pellets before (pretest; Figure 5A) and after (test; Figure 5B) mediated aversion training. As in the previous experiments, only rats that received minimal training of a CS (Group 2040) showed evidence for mediated aversion learning in the test session. Rats in Group 2040 that received CS-toxin pairings ate fewer pellets than rats that received toxin without CS presentations. Furthermore, those rats showed less consumption in the test session than the rats that were made ill after more extensive CS-food training. Although those rats also consumed fewer pellets in the pretest than the rats in the other groups (Figure 5A), perhaps because they had the least prior exposure to pellets, they showed greater reduction of consumption from that pretest baseline than the other rats (Figure 5C). Finally, there was no evidence that rats made ill after the partially-reinforced CSs showed greater mediated aversion learning than those made ill after the consistently-reinforced CS; indeed the insignificant trend was in the opposite direction.
Figure 5.
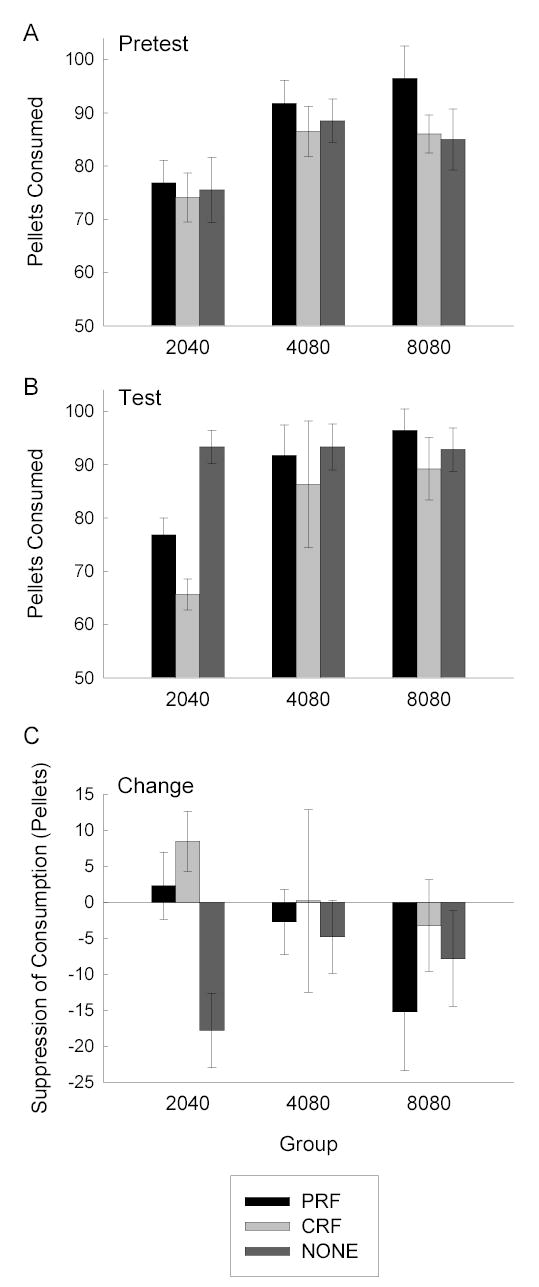
Mediated food aversion learning in Experiment 3. Panels A and B show mean (± sem) food consumption in the pretest and test sessions, respectively, and Panel C shows the mean (±sem) change in consumption (pretest minus test). In Panel C, reductions in consumption (positive change scores) reflect the acquisition of a food aversion. The bars indicate consumption of separate subgroups of rats that received pairings of either the consistently-reinforced (CRF), the partially reinforced (PRF), or no (NONE) conditioned stimulus with toxin between the pretest and test phases. The first two digits of each group name indicate the total number of CRF trials, and the second two digits indicate the total number of PRF trials received in that phase.
A group X cue identity (tone or light trained as CRF cue) X aversion cue (CRF CS, PRF CS, or no CS) ANOVA of test consumption (Figure 5B) showed significant effects of group, F(2, 42) = 6.55, and aversion cue F(2, 51) = 4.69, but not of the counterbalancing variable (cue identity), F < 1. An analysis that contrasted consumption after pairings of the CRF CS vs the PRF CS with toxin, and which excluded the rats made ill in the absence of any CS, approached significance across all three groups, F(1, 42) = 3.75, p = .06, but did not approach significance in any of the individual groups, ps > .10. Recall, however, that this nonsignificant trend was in the opposite direction as predicted by the hypothesis under investigation. Most important, a group X aversion cue analysis, which contrasted consumption in all rats that received aversion training with a CS (CRF and PRF, pooled) versus consumption of rats that received aversion training with no CS, showed a significant main effect of aversion cue, F(1, 54) = 5.78, and a significant group X aversion cue interaction, F(2, 54) = 3.24. Among individual groups, the simple effect of aversion cue (CS vs no CS), which indexed mediated learning, was significant only in Group 2040, F(1, 15) = 32.87; other groups, Fs < 1.
A group X cue identity X aversion cue ANOVA of pretest consumption showed a significant effect of group, F(2, 42) = 5.21. A post-hoc analysis (Newman-Keuls) showed that Group 2040 consumed fewer pellets than either of the other two groups. Finally, a comparable ANOVA that also included test vs pretest as a variable (thus evaluating the change in consumption between pretest and test) showed a significant effect of that variable, F(1, 42) = 5.04, as well as an effect of group, F(2, 42) = 8.80. As with the test consumption scores, an overall contrast that compared the change in consumption after pairings of either the CRF or PRF cues with toxin, which excluded the rats made ill in the absence of no CS presentations, did not approach significance, F(1, 42) = 2.05. Also as with the test consumption scores, a contrast that evaluated the change in consumption between pretest and test in rats that received either the CRF or PRF CS versus no CS was significant only in Group 2040, F(1, 51) = 10.16.
Figure 6 shows the results of the test of responding after explicit devaluation of the food reinforcer, in terms of change in responding from the final conditioning session (shown in Figure 4). Rats that had received food-LiCl pairings prior to testing showed less responding in testing (not shown) and more loss in responding (Figure 6) than rats that received food and LiCl unpaired. Although test responding was greater in groups that had received more training, neither the amount of training nor the reinforcer contingency affected the magnitude of the devaluation effect. Group (amount of training) X cue identity X devaluation contingency (paired or unpaired) ANOVAs of the change scores (last training session minus test) scores showed significant effects of group, F(2, 48) = 3.76, devaluation, F(1, 48) = 38.24, and cue identity, F(1, 48) = 13.82. No other effects or interactions were significant; notably, none of the interactions with the devaluation variable approached significance, Fs < 1. A comparable ANOVA of performance on the test session itself (not shown) led to identical conclusions. Finally, neither pre-CS responding during the test session (2.6 ± 2.1%, 2.4 ± 1.9%, and 2.6 ± 1.3% in Groups 2040, 4080, and 8080, respectively) nor change in pre-CS responding differed across groups, Fs < 1.
Figure 6.
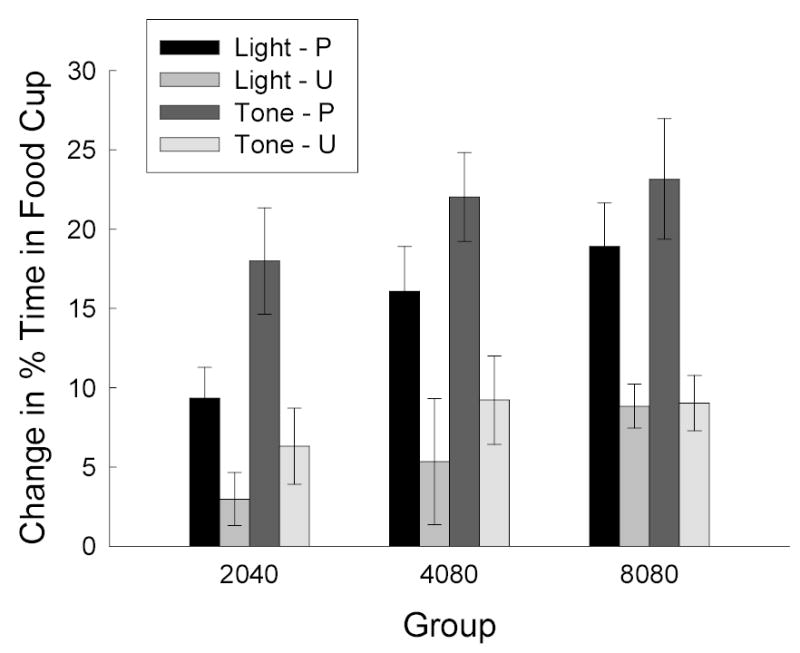
Reinforcer devaluation test in Experiment 3. The bars indicate the mean (±sem) change in food cup responding to the light and tone conditioned stimuli between the final session of training and the test session. Greater reductions in responding (greater positive change scores) reflect greater devaluation effects. The bars labeled P show change in responding after food-toxin pairings, and the bars labeled U shows change in responding after unpaired presentations of food and toxin. The first two digits of each group name indicate the total number of CRF trials, and the second two digits indicate the total number of PRF trials received in that phase.
Discussion
As in previous studies, mediated food aversion learning was observed when CS-illness pairings were administered after minimal numbers of CS-US pairings, but not after more extensive training. Furthermore, there was no evidence that the use of a partial reinforcement contingency, which should have maintained CS associability, maintained the ability of CSs to generate mediated taste aversion learning with more extensive training. Thus, Experiment 3, like Experiment 1, provided no support for the possibility that extended CS-food training prevents subsequent mediated aversion learning by reducing the associability of the CS or its associatively-activated food representation.
Nevertheless, CRs elicited by the CSs remained sensitive to devaluation of the food US by direct food-toxin pairings, regardless of the amount of training or reinforcement contingency. This outcome supports the claim that mediated aversion learning and reinforcer devaluation performance are subserved by different aspects of associatively-activated event representations.
General Discussion
The results of Experiments 1 and 3 replicated previous studies (e.g. Holland, 1981, 1990, 1998) in showing that a food aversion may be established by pairing an associatively-activated representation of that food (in the absence of the food itself) with illness. These results join others in showing that CS-activated food representations may substitute for food itself in a variety of functions, including the generation of CRs (Holland & Straub, 1979), setting the occasion for responding to other CSs (Holland & Forbes, 1982a), the extinction of previously-established food aversions (Holland & Forbes, 1982b), and overshadowing or potentiating conditioning of aversions to other stimuli (Holland, 1983).
Furthermore, these results extended Holland’s (1998) observation that this representation-mediated learning occurs only after relatively small amounts of training of the CS-food relation. This outcome was obtained with both between- groups (Experiment 3) and within-subjects (Experiment 1) designs, so it was not simply the extensive presentation of food (e.g. Adams, 1982) or repeated activation of a food representation that rendered that representation less susceptible to serving as a substitute for food in aversion learning. In Experiments 1 and 3, the same food was used as the reinforcer for both the minimally- and extensively-trained CSs. Thus, the differential susceptibility to mediated aversion learning is apparently related to experience with a CS and its particular activation of a food representation.
The primary new conclusion from these studies is that the reduced susceptibility to mediated learning with extended training is not due to general losses of associability of the CS or the food representation it activates, which may occur according to the Pearce-Hall (1980) model. Two manipulations designed to forestall such losses, the insertion of CS extinction trials after extended CS-food training but before mediated aversion training (Experiment 1), and the use of partial reinforcement procedures during CS-US training (Experiment 3), did not facilitate mediated aversion learning.
At the same time, consistent with the predictions of the Pearce-Hall (1980) model, the results of Experiment 2 demonstrated that the insertion of CS extinction trials indeed enhanced or restored CS associability after extensive training of that CS. Although the introduction of a brief CS-extinction phase after extensive CS-food training significantly reduced conditioned responding, it enhanced the subsequent acquisition of new learning to that CS when it was paired with a new food reinforcer. Thus, the results of Experiment 2 extended the findings of Hall and Pearce (1979) to an appetitive conditioning procedure. Furthermore, because the training and surprise (extinction) procedures used in Experiments 1 and 2 were identical, this outcome shows that the procedure used in Experiment 1 to restore CS associability was indeed effective. Thus, the lack of any facilitatory effect of that manipulation on mediated aversion learning in Experiment 1 implies that losses in CS associability were not responsible for the resistance to mediated aversion learning after extended CS-food training observed in that study.
Of course, it could be argued that despite the effectiveness of this surprise procedure in restoring the associability of the CS itself in Experiment 2, it is insufficient to restore the associability of the CS-activated food representation. Likewise, it is possible that surprise effects of this sort are reinforcer-specific. In Experiment 2, both the omitted surprising event and the reinforcer for new learning were foods, whereas in Experiment 1, the reinforcer for new learning was a toxin. Although it may seem arbitrary to assume that whereas losses in CS associability are reinforcer-general, recovery from those losses are reinforcer-specific, there is precedent for these assumptions in the literature of unblocking with downshifts in the number of reinforcers delivered (Dickinson & Mackintosh, 1976).
All in all, the results of the present study indicate that the ability of briefly-trained CSs to mediate taste aversion learning to their reinforcers is not the result of greater associability of those CSs. However, beyond the rejection of this hypothesis, these experiments shed relatively little light on the origins of this amount of training effect. Nevertheless, it is instructive to consider alternative accounts. One such account is based on changes in processing of the US, rather than of the CS. Although the same physical food event followed all the CSs in these experiments, it may have been processed or represented differently when delivered after briefly-trained CSs than when it was presented after extensively-trained CSs. Perhaps the simplest version of this view is that, consistent with models like the Rescorla-Wagner (1972) model, after the briefly-trained CS, the US is processed more extensively because it is less expected. That more extensive processing of the US may include activation of sensory-perceptual processes that could mediate learned taste aversions, processing that might be absent when a well-predicted food US is delivered after an extensively-trained CS.
From this perspective, the critical determinant of mediated learning potential is not the amount of training per se but rather the greater processing of surprising USs that is guaranteed by small amounts of training. Thus, if the surprise value of food presentations could be maintained despite extensive training of a CS, that CS might be able to mediate aversion learning. At first glance, it would appear that the partial reinforcement of a CS (as in Experiment 3) should maintain processing of the US on reinforced trials over larger number of US presentations, and hence should enhance mediated learning. We found no such evidence. However, it is notable that the PRF and CRF schedules supported similar, high levels of CRs after extended training in Experiment 3, which suggests that the expected value of the US did not differ much between those two schedules. Thus, on reinforced trials, the US would have been similarly processed in both conditions. Note too that the high level of US expectancy in the PRF condition would tend to maximize the surprise effect of the omission of the US on nonreinforced trials in the PRF condition, increasing the chance of enhancement of processing of the CS on those trials, as was intended in Experiment 3, and as was observed by Pearce and his colleagues (Collins & Pearce, 1985; Collins, Young, Davies, & Pearce, 1983). Perhaps extensive training with a lean schedule of partial reinforcement, or indeed any manipulation designed to keep the associative strength of a CS low, might permit the maintenance of mediated learning ability over extended training of a CS. Likewise, this ability might be restored to an extensively-trained CS, if the US were made surprising again. For example, an extensively-trained CS might be reinforced while accompanied by a separately trained conditioned inhibitor, or after an equally extensive period of extinction. On the other hand, if sensory aspects of CS-food learning are retained in extinction (e.g., Rescorla, 1996a), the CS might not reacquire this ability, despite the overall surprise value of the reinstated reinforcer, because the sensory aspects of that reinforcer would remain expected.
Regardless of the mechanisms by which it occurs, the change in the ability of associatively-activated event representations to participate in new learning observed here over the course of training is consistent with the claims of many other researchers who have suggested that the contents of learning change over the course of training. Common to most of these views is the notion that learned performance is more flexible and goal-oriented in early stages but becomes increasing automatic and less governed by its consequences with more extended training (e.g., Allport, 1937; Kimble & Perlmuter, 1970; Tolman, 1948.) For example, Adams and Dickinson (1981) suggested that extended training may be accompanied by a shift from behavioral control by stimulus-reinforcer or response-reinforcer associations to stimulus-response associations. This view is echoed in several recent investigations of the neurobiological bases of learning. For example, the results of a series of investigations by Packard and his colleagues (e.g. Packard & McGaugh, 1996; Poldrack & Packard, 2003) indicate that the behavior of rats in plus mazes is controlled primarily by hippocampus-dependent learning of place-food relations early in training, but becomes more controlled by caudate-dependent stimulus-response learning as training progresses. Similarly, Bussey et al (1996) attributed initial stimulus-reinforcer learning in a conditional discrimination task to anterior cingulate systems, and later stimulus-response learning to posterior cingulate systems.
Implicit in many of these views is the idea that CSs lose access to stimulus aspects of a memorial representation of the reinforcer as training progresses. Within the context of the present results, early in training the CS activates a representation of food that not only mediates the performance of food-related CRs, but also can substitute for food itself in the establishment of a learned aversion; later in training the CS no longer activates such a representation. However, in both Experiment 3 and in Holland (1998, Exp. 3), despite the inability of extensively-trained CSs to produce mediated aversion learning, those CSs clearly maintained their associative access to a representation of food, because their food-cup approach CRs were still sensitive to reinforcer devaluation. Thus, the notion that the CSs activate a unitary “food representation” seems untenable. Instead it seems reasonable to suggest that reinforcer devaluation and representation-mediated learning effects involve different aspects of associatively-activated event representations, and that associative access to those various representational features changes over the course of training.
Holland (1990, 1998) proposed that representation-mediated learning and representation-mediated performance of CRs (as illustrated in reinforcer devaluation effects) reflect the associative activation of perceptual and motivational processing systems, respectively. In an extreme version of the former case, Holland (1990) suggested that mediated food aversion learning might occur because the CS activates perceptual processing normally activated by the food itself, as if for example, the tone induced tasting of the food reinforcer. Holland (1990) presented initial behavioral evidence to support that claim, recording oral-facial taste reactivity measures (Berridge, 2001; Grill & Norgen, 1978). In one experiment (Holland, 1990, Exp. 1a), thirsty rats first received pairings of two auditory CSs with the delivery of two differently-flavored sucrose solutions to a liquid cup in a standard experimental chamber. Then, an aversion to one of those solutions was formed by pairing it with LiCl injection in the absence of the CSs, in the rats’ home cages. Finally, the rats were allowed to drink plain water in the liquid cups, and each of the auditory CSs was presented. The rats displayed more aversive oral/facial responses characterized as “disgust” or “disliking” responses when the auditory CS whose flavor associate had been devalued was presented than in the presence of the other CS or no CS, and more appetitive or “liking” responses when the auditory CS whose flavor associate had not been devalued was presented. Because these responses are often claimed to reflect reactivity to taste properties of stimuli and are not necessarily correlated with the amount of consumption (Berridge, 2001), we suggested that their emergence to plain water in the presence of the two auditory CSs reflected associatively-activated “tasting”.
A second experiment (Holland, 1990, Exp. 3) provided more compelling evidence for this associative control of perceptual taste processing by auditory CSs. Water-deprived rats first received pairings of tone and noise CSs with sucrose or saline solutions. Next, in their home cages the rats were trained with a negative patterning taste aversion discrimination procedure, in which each flavor alone was paired with toxin, but a sucrose+saline compound was nonreinforced. Finally, rats were allowed to drink plain water in the experimental chambers. As with the CS whose flavor associate had been devalued in the previous experiment, when either CS was presented alone, the rats displayed aversive responses, as would be appropriate to the individual sucrose and saline solutions. However, when for the first time, the noise and tone were presented simultaneously, the rats displayed predominantly appetitive responses, as would be appropriate to the taste of the sucrose+saline compound. It is difficult to interpret this pattern of results without recourse to some control of taste processing by the auditory CSs alone.
In a more recent experiment (Kerfoot et al, 2004; unpublished data), similar to that of Holland (1990, Exp. 1a), we provided some neural systems evidence for this notion. In a single training session, food-deprived rats first received a tone CS and the delivery of a sucrose solution through an intra-oral cannula. For some of these rats, the tone and sucrose were paired and for others those events were explicitly unpaired. Then, half of the rats in each of those groups were made ill immediately after intra-oral infusions of sucrose alone and the rest were made ill 6–8 hours later. Finally, in a subsequent session, the tone was presented with intra-oral infusion of plain water in all rats. The rats that previously received tone-sucrose and then sucrose-illness pairings displayed aversive responses, whereas the rats that had received tone-sucrose pairings but unpaired sucrose and illness displayed appetitive responses. Rats that had not received tone-sucrose pairings displayed neither appetitive nor aversive responses in the test. The perceptual origin of these responses was suggested by the results of an analysis of patterns of brain activation. Immediately after the test sessions, the rats were sacrificed and their brains assayed for expression of FOS protein as an indicant of neural activity during the tone/water test. More FOS was expressed in gustatory sensory regions in the rats that had previously received tone-sucrose pairings, regardless of devaluation training. By contrast, more FOS was observed in brain regions associated with appetitive and aversive taste reactivity (anterior and posterior accumbens shell, respectively; Reynolds & Berridge, 2000) in the tone-sucrose, nondevalued, and tone-sucrose, devalued rats, respectively. It would be valuable to determine whether these devaluation-sensitive taste-reactivity responses to the tones would also be observed only after minimal training, as given in these studies, and not after more extended tone-sucrose training. Finally, it is notable that analogous findings have been reported in humans; for example, in an fMRI study, McIntosh, Cabeza, and Lobaugh (1998) reported activation of primary visual cortex by auditory cues that were consistently paired with visual stimuli. Interestingly, these authors did not report that the participants actually experienced the predicted but absent auditory cues, a finding reported in some earlier studies (Howells, 1944; Leuba, 1940). Thus, the extreme stance (e.g., following Konorski’s, 1967, p. 174, discussion of CS-elicited “hallucinations” of USs) that the rats in our studies actually “tasted” the absent flavors is unnecessary as long as the CSs controlled neural processing that permitted association of associatively-activated sensory information with illness.
By contrast, representation-mediated performance of food cup entry responses, as evidenced in devaluation procedures, which persists over extended training, may reflect a CS’s continued access to more downstream aspects of US processing systems. Because such devaluation effects are CS- and US-specific (e.g. Holland, 1990, 1998; Colwill & Motzkin, 1994) these incentive motivational systems must include the association of sensory properties with systems that attach motivational significance to events (e.g., Balleine & Dickinson, 2000; Blundell et al., 2001), but may not provoke sensory processing sufficient to mediate learning about those absent events. For example, in a recent model of amygdala-cortical interactions in outcome-expectancy learning based in part on the results of electrophysiological recordings of neuronal activity and devaluation experiments (Holland & Gallagher, 2004), neurons in the basolateral amygdala that encode specific outcome expectancies are needed to “train” similar coding in neuron in the orbitofrontal cortex. These cortical neurons may later guide performance, independent of the amygdala. Within such models, the potential exists for the re-encoding of sensory information in systems that mediate performance but which may not support the kinds of mediated learning discussed in this article.
Acknowledgments
I thank Erin Kerfoot and Vanessa McKenna for their technical assistance.
Footnotes
This research was supported by grant MH65879 from the National Institutes of Health.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Quarterly Journal of Experimental Psychology. 1982;34B:77–98. [Google Scholar]
- Adams, C. & Dickinson, A. (1981). Actions and habits: Variations in associative representations during instrumental learning. In N. E. Spear & R. R. Miller (eds.), Information Processing in Animals: Memory Mechanisms (pp. 143–165). Hillsdale, N. J.: Erlbaum.
- Allport, G. W. (1937). Personality: A psychological interpretation New York: Holt.
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The effects of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive memory. Journal of Neuroscience. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K.C. (2001). Reward learning: reinforcement, incentives, and expectations. In D. Medin (Ed.), The Psychology of Learning and Motivation, 40, 223–278. San Diego, CA: Academic Press.
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupts selective aspects of reinforcer representation in rats. Journal of Neuroscience. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Pearce JM. Predictive accuracy and the effects of partial reinforcement on serial autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:548–564. [Google Scholar]
- Collins L, Young DB, Davies K, Pearce JM. The influence of partial reinforcement on serial autoshaping with pigeons. Quarterly Journal of Experimental Psychology. 1983;35B:275–290. doi: 10.1080/14640748308400893. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior. 1994;22:384–394. [Google Scholar]
- Dickinson A, Mackintosh NJ. Classical conditioning in animals. Annual Review of Psychology. 1978;29:587–612. doi: 10.1146/annurev.ps.29.020178.003103. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Learning about cues in their absence: Evidence from flavour preferences and aversions. Quarterly Journal of Experimental Psychology. 2003;56B:56–67. doi: 10.1080/02724990244000160. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Hall G. Learning about associatively-activated stimulus representations: Implications for acquired equivalence and perceptual learning. Animal Learning & Behavior. 1996;24:233–255. [Google Scholar]
- Hall G, Pearce JM. Latent inhibition of a CS during CS-US pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:31–42. [PubMed] [Google Scholar]
- Holland PC. Acquisition of representation mediated conditioned food aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Representation mediated overshadowing and potentiation of conditioned aversions. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:1–13. [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC. Amount of training affects associatively-activated event representation. Neuropharmacology. 1998;37:461–469. doi: 10.1016/s0028-3908(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bashaw M, Quinn J. Amount of training and stimulus salience affects associability changes in serial conditioning. Behavioural Processes. 2002;59:169–183. doi: 10.1016/s0376-6357(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Forbes DT. Control of conditional discrimination performance by CS evoked event representations. Animal Learning & Behavior. 1982;10:249–256. (a) [Google Scholar]
- Holland PC, Forbes DT. Representation mediated extinction of flavor aversions. Learning and Motivation. 1982;13:454–471. (b) [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first and second order appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Howells TH. The experimental development of color-tone synesthesia. Journal of Experimental Psychology. 1944;34:87–103. [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:90–109. [PubMed] [Google Scholar]
- Kerfoot, E.C., Lee, H.J., Agarwal, I., Holland, P.C. (2004). Conditioned auditory stimuli control appetitive and aversive taste-reactivity responses in a devaluation task. Society for Neuroscience Abstract 206.10 [DOI] [PMC free article] [PubMed]
- Kimble GA, Perlmuter LC. The problem of volition. Psychological Review. 1970;77:361–384. doi: 10.1037/h0029782. [DOI] [PubMed] [Google Scholar]
- Konorski, J. (1967). Integrative activity of the brain Chicago: University of Chicago Press.
- Leuba C. Images as conditioned sensations. Journal of Experimental Psychology. 1940;26:345–351. [Google Scholar]
- McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. Journal of Neurophysiology. 1998;80:2790–2796. doi: 10.1152/jn.1998.80.5.2790. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Collins L. An evaluation of the associative strength of a partially reinforced serial CS. Quarterly Journal of Experimental Psychology. 1987;39B:273–293. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;106:532–552. [PubMed] [Google Scholar]
- Pearce JM, Kaye H, Collins L. A comparison of the effects of partial reinforcement schedules using a within-subject serial autoshaping procedure. Quarterly Journal of Experimental Psychology. 1985;37B:379–396. [Google Scholar]
- Pearce JM, Redhead ES, Aydin A. Partial reinforcement in appetitive Pavlovian conditioning with rats. Quarterly Journal of Experimental Psychology. 1997;50B:273–294. doi: 10.1080/713932660. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Response outcome versus outcome response associations in instrumental learning. Animal Learning & Behavior. 1992;20:223–232. [Google Scholar]
- Rescorla RA. Preservation of Pavlovian associations through extinction. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1996;49:245–258. (a) [Google Scholar]
- Rescorla RA. Response-outcome associations remain functional through interference treatments. Animal Learning & Behavior. 1996;24:450–458. (b) [Google Scholar]
- Rescorla RA. Within-subject partial reinforcement extinction effect in autoshaping. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiology. 1999;52:75–87. [Google Scholar]
- Rescorla, R. A. & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In A. H. Black & W. F. Prokasy (Eds.), Classical Conditioning II, (64 99). New York: Appleton Century Crofts.
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychological Review. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Trapold, M. A., & Overmier, J. B. (1972). The second learning process in instrumental training. In A. Black & W. F. Prokasy (Eds.), Classical Conditioning II (427–452). New York: Appleton-Century-Crofts.


