Abstract
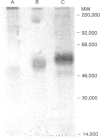
The bovine interleukin-2 receptor-alpha (IL-2R alpha) gene has been isolated and a rabbit antiserum against a fusion protein of the gene has been produced. However, the antiserum does not inhibit IL-2-dependent proliferation. Since a panel of monoclonal antibodies (mAb) to bovine activation antigens was available, we transfected the gene into mouse L fibroblasts, selected stable transfectants with the rabbit antiserum, and screened for antibodies that bound the transfected cells but not the untransfected cells. Three mAb were selected and all three precipitated a molecule of M(r) 55,000 (under reducing conditions) from activated cells, as expected from homology with mouse and human IL-2R alpha (CD25, Tac). One of the three mAb was a strong inhibitor of IL-2-dependent proliferation of bovine lymphocytes. Thus, the availability of transfected cells allowed us to establish quickly and unequivocally the antigenic specificity of a number of antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R., Scher I., Sharrow S. O., Shevach E. M. Non-activated guinea-pig T cells and thymocytes express Ia antigens: FACS analysis with alloantibodies and monoclonal antibodies. Immunology. 1984 Jan;51(1):93–102. [PMC free article] [PubMed] [Google Scholar]
- Deeg H. J., Wulff J. C., DeRose S., Sale G. E., Braun M., Brown M. A., Springmeyer S. C., Martin P. J., Storb R. Unusual distribution of Ia-like antigens on canine lymphocytes. Immunogenetics. 1982;16(5):445–457. doi: 10.1007/BF00372103. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D. A., Prospero T. D., Roditi I. J., Kelke C., Baumann I., Eichhorn M., Williams R. O., Ahmed J. S., Baldwin C. L., Clevers H. Expression of Tac antigen component of bovine interleukin-2 receptor in different leukocyte populations infected with Theileria parva or Theileria annulata. Infect Immun. 1990 Dec;58(12):3847–3855. doi: 10.1128/iai.58.12.3847-3855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Heyes J., Austin P., Bodmer J., Bodmer W., Madrigal A., Mazzilli M. C., Trowsdale J. Monoclonal antibodies to HLA-DP-transfected mouse L cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3417–3421. doi: 10.1073/pnas.83.10.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter W., Goldman C. K., Casabo L., Nelson D. L., Greene W. C., Waldmann T. A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol. 1987 May 1;138(9):2917–2922. [PubMed] [Google Scholar]
- Hori T., Uchiyama T., Onishi R., Kamio M., Umadome H., Tamori S., Motoi T., Kodaka T., Uchino H. Characteristics of the IL-2 receptor expressed on large granular lymphocytes from patients with abnormally expanded large granular lymphocytes. Implication of a non-Tac IL-2-binding peptide. J Immunol. 1988 Jun 15;140(12):4199–4203. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Greene W. C. Contrasting interleukin 2 binding properties of the alpha (p55) and beta (p70) protein subunits of the human high-affinity interleukin 2 receptor. J Exp Med. 1987 Oct 1;166(4):1156–1161. doi: 10.1084/jem.166.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Wijffels G. L., Mackay I. R., Walker I. D. Characterization of a 95,000 molecule on sheep leucocytes homologous to murine Pgp-1 and human CD44. Immunology. 1988 Sep;65(1):93–99. [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naessens J. Characterisation of lymphocyte populations in African buffalo (Syncerus caffer) and waterbuck (Kobus defassa) with workshop monoclonal antibodies. Vet Immunol Immunopathol. 1991 Jan;27(1-3):153–162. doi: 10.1016/0165-2427(91)90094-s. [DOI] [PubMed] [Google Scholar]
- Naessens J., Newson J., McHugh N., Howard C. J., Parsons K., Jones B. Characterization of a bovine leucocyte differentiation antigen of 145,000 MW restricted to B lymphocytes. Immunology. 1990 Apr;69(4):525–530. [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985 Jan;134(1):37–44. [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Saiki O., Doi S., Fuji M., Sugamura K., Hara H., Negoro S., Kishimoto S. Novel receptor-mediated internalization of interleukin 2 in B cells. J Immunol. 1988 Feb 1;140(3):866–870. [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. D., Shaw J., Paetkau V., Bleackley R. C., Magnuson N. S., Reeves R., Magnuson J. A. Cloning of cDNA for the bovine IL-2 receptor (bovine Tac antigen). Immunology. 1988 Apr;63(4):603–610. [PMC free article] [PubMed] [Google Scholar]