Abstract
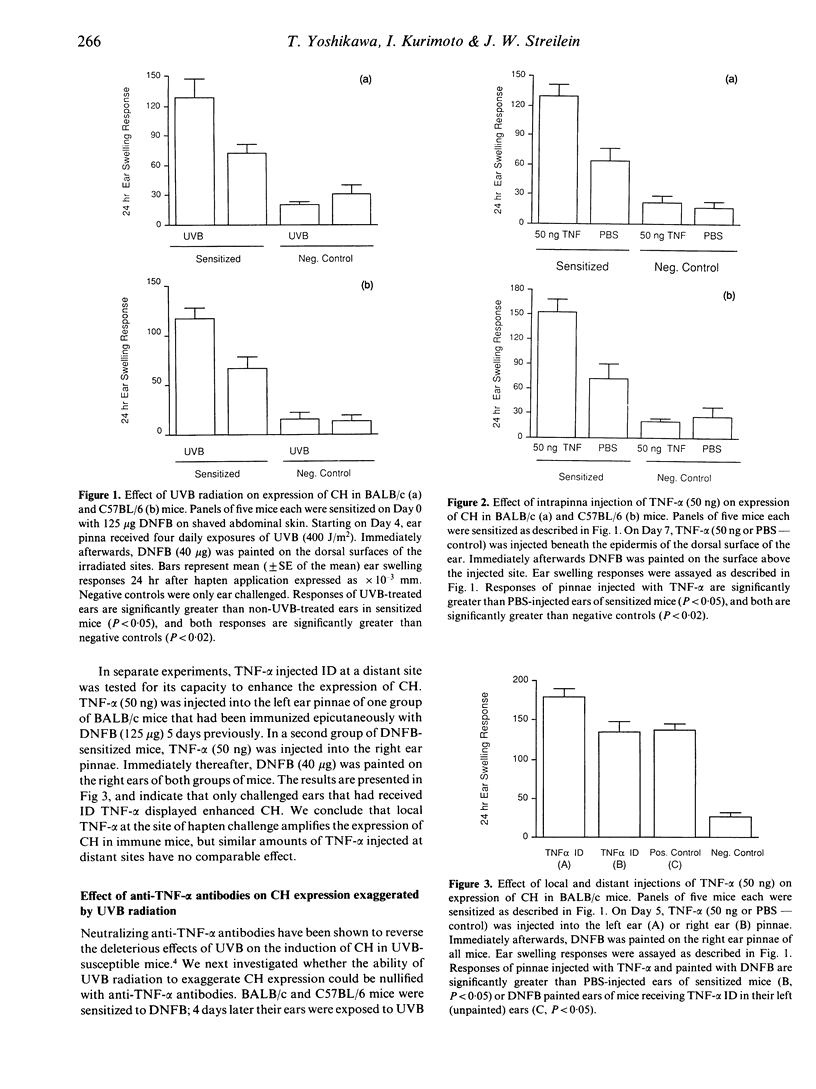
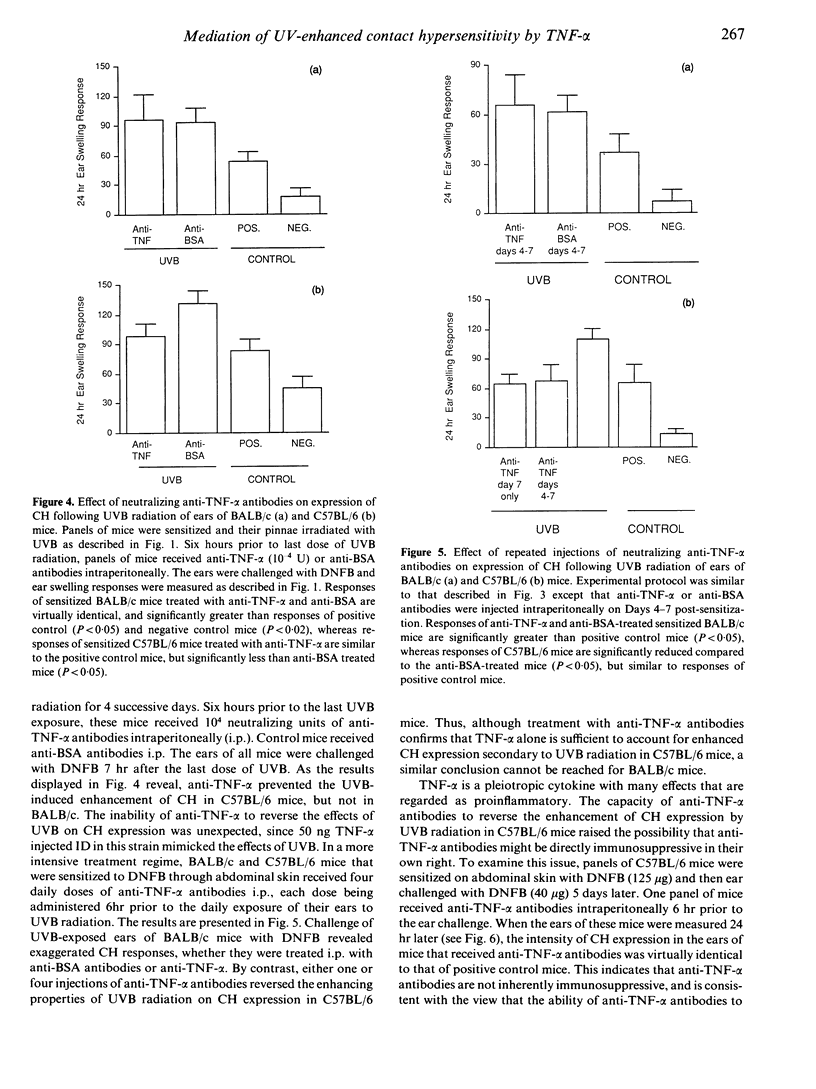
Acute, low-dose ultraviolet B radiation (UVB) impairs the induction of contact hypersensitivity (CH) to dinitrochlorobenzene (DNCB) in certain inbred strains of mice (termed UVB-susceptible), but not in others (termed UVB-resistant). By contrast, exposure of mouse ear skin to an identical regimen of UVB has been reported to exaggerate the expression of CH. Recently, tumour necrosis factor-alpha (TNF-alpha) has been demonstrated to mediate the deleterious effects of UVB on CH induction, presumably through local release of TNF-alpha within UVB exposed skin. The present studies were conducted to determine whether TNF-alpha also mediates the exaggerated expression of CH induced by UVB radiation. It was found that TNF-alpha, injected intradermally at the ear challenge site, enhanced the expression of CH to DNFB in conventionally sensitized mice. Interestingly, TNF-alpha was able to amplify the expression of CH in the ears of both UVB-susceptible strains of mice, and UVB-resistant strains. However, anti-TNF-alpha antibodies neutralized UVB-enhanced CH in UVB-susceptible mice, but not in UVB-resistant mice. These findings support the proposition that TNF-alpha, released from UVB-exposed epidermal cells, is a critical mediator of the effects of UVB radiation on induction and expression of contact hypersensitivity. The effects of UVB radiation, intradermal (ID) TNF-alpha, and/or epicutaneously applied DNFB on epidermal Langerhans' cells were also evaluated and compared. Whereas epicutaneously applied DNFB alone profoundly depleted the epidermis of Langerhans' cells, DNFB painted on UVB-exposed or TNF-alpha-treated skin was much less effective at eliminating normal appearing Langerhans' cells. These results suggest that one direct effect of TNF-alpha on Langerhans' cells may be to immobilize these antigen-presenting cells transiently within the epidermis. It is proposed that this immobilization has the paradoxical effect (a) of interfering with sensitization, by preventing hapten-bearing Langerhans' cells from migrating to the draining lymph node, while at the same time (b) of amplifying CH expression by lengthening the interval of hapten retention and presentation with the epidermis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Hunziker N., Winkelmann R. K. Langerhans cells in contact dermatitis of the guinea pig. Arch Dermatol. 1978 Sep;114(9):1309–1313. [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. H., Gurish M. F., Daynes R. A. Relationship between epidermal Langerhans cell density ATPase activity and the induction of contact hypersensitivity. J Immunol. 1981 May;126(5):1892–1897. [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher E., Chase M. W. Studies on the sensitization of animals with simple chemical compounds. XII. The influence of excision of allergenic depots on onset of delayed hypersensitivity and tolerance. J Exp Med. 1969 Jan 1;129(1):103–121. doi: 10.1084/jem.129.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Lyons M. B., Middleton M. H., Yohn J. J., Kashihara-Sawami M. Ultraviolet radiation can either suppress or induce expression of intercellular adhesion molecule 1 (ICAM-1) on the surface of cultured human keratinocytes. J Invest Dermatol. 1990 Aug;95(2):132–138. doi: 10.1111/1523-1747.ep12477877. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Hauser C., Vassalli P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J Exp Med. 1991 Mar 1;173(3):673–679. doi: 10.1084/jem.173.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Sharpe R. J., Margolis R. J., Askari M., Amento E. P., Granstein R. D. Induction of dermal and subcutaneous inflammation by recombinant cachectin/tumor necrosis factor (TNF alpha) in the mouse. J Invest Dermatol. 1988 Oct;91(4):353–357. doi: 10.1111/1523-1747.ep12475754. [DOI] [PubMed] [Google Scholar]
- Sreilein J. W. Antigen-presenting cells in the induction of contact hypersensitivity in mice: evidence that Langerhans cells are sufficient but not required. J Invest Dermatol. 1989 Oct;93(4):443–448. doi: 10.1111/1523-1747.ep12284018. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Bergstresser P. R. Genetic basis of ultraviolet-B effects on contact hypersensitivity. Immunogenetics. 1988;27(4):252–258. doi: 10.1007/BF00376119. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Bergstresser P. R., Streilein J. W. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980 Jan;124(1):445–453. [PubMed] [Google Scholar]
- Toews G. B., Bergstresser P. R., Streilein J. W. Langerhans cells: sentinels of skin associated lymphoid tissue. J Invest Dermatol. 1980 Jul;75(1):78–82. doi: 10.1111/1523-1747.ep12521270. [DOI] [PubMed] [Google Scholar]
- Vermeer M., Streilein J. W. Ultraviolet B light-induced alterations in epidermal Langerhans cells are mediated in part by tumor necrosis factor-alpha. Photodermatol Photoimmunol Photomed. 1990 Dec;7(6):258–265. [PubMed] [Google Scholar]
- Weiser W. Y., Temple P. A., Witek-Giannotti J. S., Remold H. G., Clark S. C., David J. R. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Streilein J. W. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphism at the Tnfa and Lps loci governs susceptibility. Immunogenetics. 1990;32(6):398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Streilein J. W. Tumor necrosis factor-alpha and ultraviolet B light have similar effects on contact hypersensitivity in mice. Reg Immunol. 1990;3(3):139–144. [PubMed] [Google Scholar]


