Abstract
The CD45 or leucocyte-common antigens are encoded by a single gene but can be found in various forms due to alternative splicing of three exons near the 5' end of the gene. The CD45 antigens are major glycoproteins of all types of leucocytes. Monoclonal antibodies recognizing restricted epitopes of CD45 have been used to distinguish phenotypic and functional subsets of lymphocytes. To facilitate epitope mapping and biochemical studies, we have expressed the extracellular portions for four different isoforms of rat CD45 in Chinese hamster ovary cells. Constructs were prepared to give four soluble CD45 isoforms, with sequence incorporating either all three alternative exons (sCD45.ABC), the B exon (sCD45.B), the C exon (sCD45.C), or no alternative exons (sCD45.O). These were expressed at approximately 5 mg/l of spent tissue culture supernatant and were antigenically active with monoclonal antibodies (mAb) that recognize all CD45 isoforms. The MRC OX22 and OX32 mAb have been used to split rat CD4+ T cells into functionally distinct subpopulations and the epitopes for these were mapped to the product of exon C. The epitope for MRC OX33, a marker for B cells, requires expression of either the A exon or the A/B exon junction. Electron microscopy showed that the extra segments contributed to an extended structure as has been predicted from the sequence. The shape of the molecule is discussed with regard to other molecules at the leucocyte cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Arthur R. P., Mason D. T cells that help B cell responses to soluble antigen are distinguishable from those producing interleukin 2 on mitogenic or allogeneic stimulation. J Exp Med. 1986 Apr 1;163(4):774–786. doi: 10.1084/jem.163.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N., Jackson D. I., Willis A. C., Williams A. F. Lymphocyte specific heterogeneity in the rat leucocyte common antigen (T200) is due to differences in polypeptide sequences near the NH2-terminus. EMBO J. 1987 May;6(5):1259–1264. doi: 10.1002/j.1460-2075.1987.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bottomly K., Luqman M., Greenbaum L., Carding S., West J., Pasqualini T., Murphy D. B. A monoclonal antibody to murine CD45R distinguishes CD4 T cell populations that produce different cytokines. Eur J Immunol. 1989 Apr;19(4):617–623. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- Cockett M. I., Bebbington C. R., Yarranton G. T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (N Y) 1990 Jul;8(7):662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991 Apr;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J., Somoza C., Killeen N., Williams A. F. Protein sequence and gene structure for mouse leukosialin (CD43), a T lymphocyte mucin without introns in the coding sequence. Eur J Immunol. 1990 Apr;20(4):875–881. doi: 10.1002/eji.1830200424. [DOI] [PubMed] [Google Scholar]
- Davis S. J., Ward H. A., Puklavec M. J., Willis A. C., Williams A. F., Barclay A. N. High level expression in Chinese hamster ovary cells of soluble forms of CD4 T lymphocyte glycoprotein including glycosylation variants. J Biol Chem. 1990 Jun 25;265(18):10410–10418. [PubMed] [Google Scholar]
- Jackson D. I., Barclay A. N. The extra segments of sequence in rat leucocyte common antigen (L-CA) are derived by alternative splicing of only three exons and show extensive O-linked glycosylation. Immunogenetics. 1989;29(5):281–287. doi: 10.1007/BF00352837. [DOI] [PubMed] [Google Scholar]
- Johnson P., Greenbaum L., Bottomly K., Trowbridge I. S. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J Exp Med. 1989 Mar 1;169(3):1179–1184. doi: 10.1084/jem.169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Barclay A. N., Mason D. W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991 May;21(5):1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Green N. M., Eason P., Yamada S. S., Yamada K. M. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988 Dec 20;7(13):4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R., Sánchez-Madrid F. Glycosylation of CD45: carbohydrate composition and its role in acquisition of CD45R0 and CD45RB T cell maturation-related antigen specificities during biosynthesis. Eur J Immunol. 1990 Dec;20(12):2667–2671. doi: 10.1002/eji.1830201221. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Alternative use of 5' exons in the specification of Ly-5 isoforms distinguishing hematopoietic cell lineages. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5364–5368. doi: 10.1073/pnas.84.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparshott S. M., Bell E. B., Sarawar S. R. CD45R CD4 T cell subset-reconstituted nude rats: subset-dependent survival of recipients and bi-directional isoform switching. Eur J Immunol. 1991 Apr;21(4):993–1000. doi: 10.1002/eji.1830210420. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Matsuyama T., Morimoto C., Schlossman S. F., Saito H. Identification of the sequence required for expression of the 2H4 epitope on the human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1567–1572. doi: 10.1084/jem.166.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Terry L. A., Brown M. H., Beverley P. C. The monoclonal antibody, UCHL1, recognizes a 180,000 MW component of the human leucocyte-common antigen, CD45. Immunology. 1988 Jun;64(2):331–336. [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
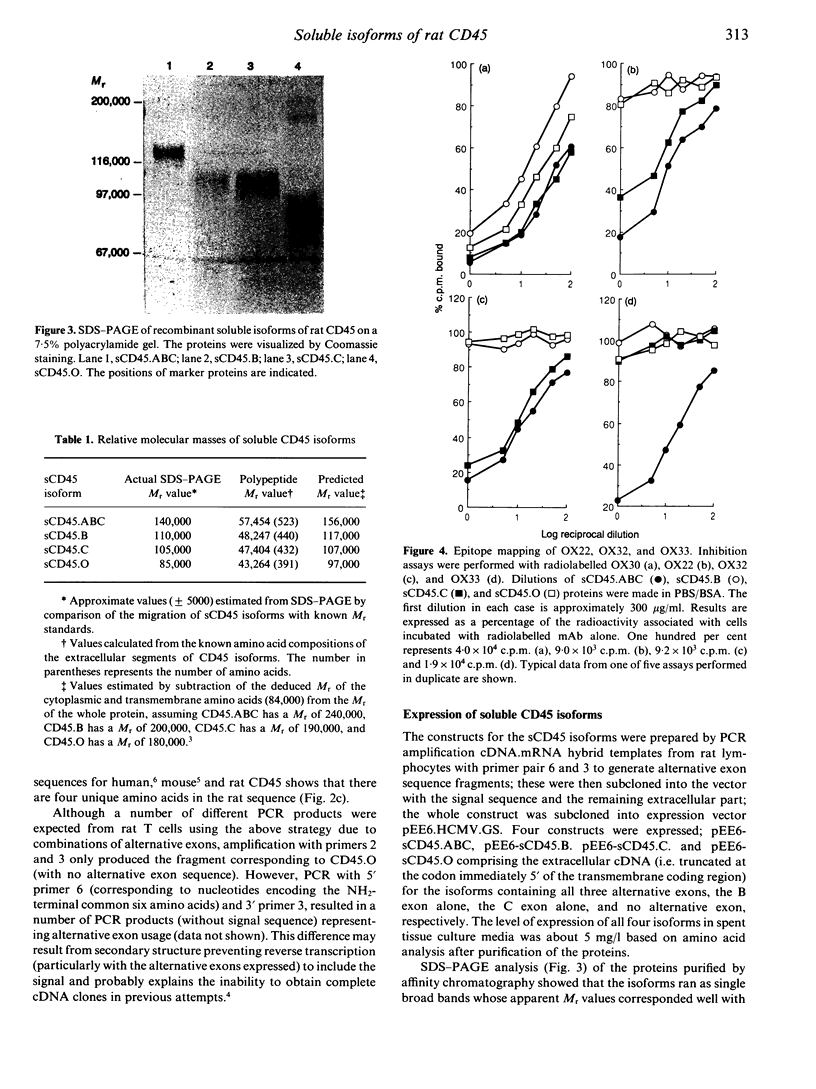
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Williams A. F., Shotton D. M. Visualisation by low-angle shadowing of the leucocyte-common antigen. A major cell surface glycoprotein of lymphocytes. EMBO J. 1985 Nov;4(11):2827–2830. doi: 10.1002/j.1460-2075.1985.tb04010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]