Abstract
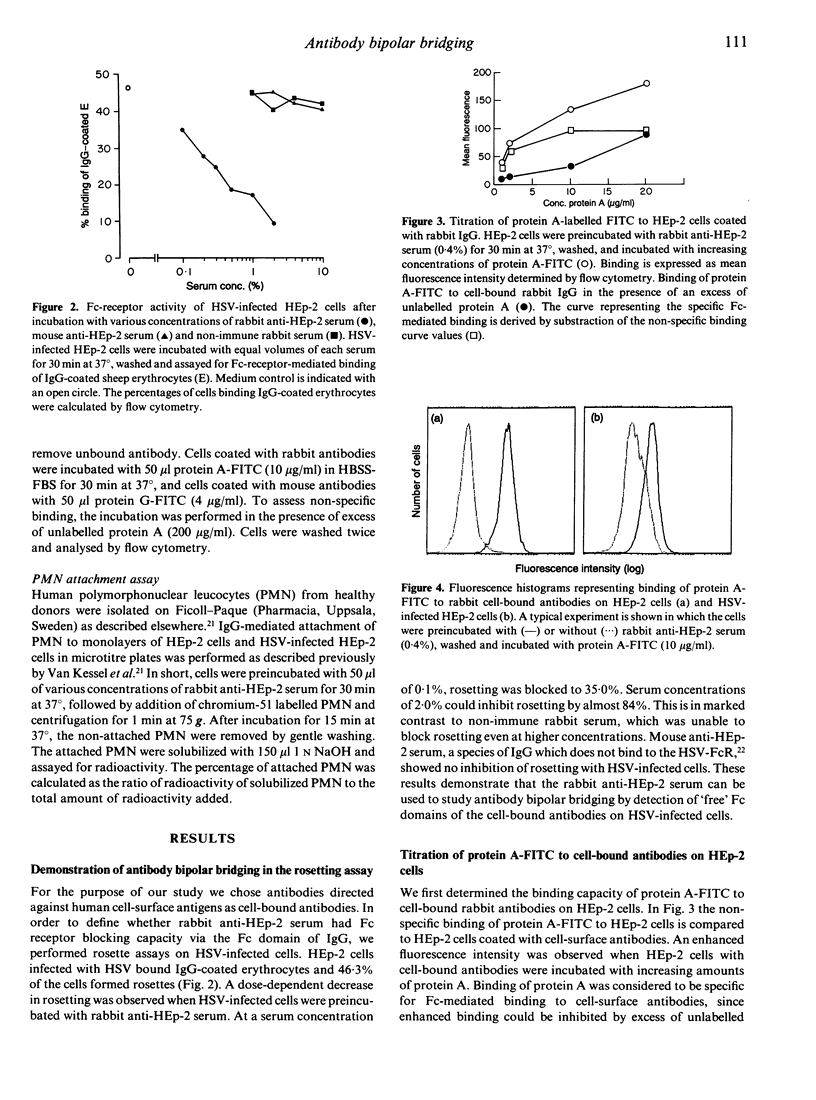
Cells infected with herpes simplex virus type 1 (HSV-1) express a cell-surface receptor able to bind the Fc portion of immunoglobulin G (IgG). In this study we provide direct evidence that bipolar bridging of antibodies, bound to the surface antigens on HSV-infected cells and to the Fc-receptor through the Fc part, offers the virus a survival advantage. Evidence was obtained by comparing the binding of FITC-labelled protein A, which has a similar binding site on IgG as the HSV-FcR, to cell-bound antibodies on HSV-infected cells and non-infected cells. The effectiveness of antibody bipolar bridging was dependent on the concentration of cell-bound IgG. At low concentrations of serum (0.1%) an 80% reduction in protein A-FITC binding to HSV-infected cells compared to non-infected cells was found. Even at higher concentrations of serum, antibody bipolar bridging resulted in a 40% reduction in the number of 'free' available Fc parts on HSV-infected cells compared to non-infected cells. Furthermore, these findings could be confirmed in a functional assay. The Fc-mediated attachment of granulocytes was significantly lower in HSV-infected cells compared to non-infected cells. From this study we conclude that HSV-FcR, by binding immune IgG in a bipolar fashion, provides the virus with an effective defence mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985 Oct;135(4):2589–2592. [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G., Voisin G. A. Antibody bipolar bridging: isotype-dependent signals given to guinea pig alveolar macrophages by anti-MHC alloantibodies. Cell Immunol. 1987 May;106(2):304–317. doi: 10.1016/0008-8749(87)90174-2. [DOI] [PubMed] [Google Scholar]
- Daëron M., Voisin G. A. H-2 antigens, on mast cell membrane, as target antigens for anaphylactic degranulation. Cell Immunol. 1978 May;37(2):467–472. doi: 10.1016/0008-8749(78)90214-9. [DOI] [PubMed] [Google Scholar]
- Dowler K. W., Veltri R. W. In vitro neutralization of HSV-2: inhibition by binding of normal IgG and purified Fc to virion Fc receptor (FcR). J Med Virol. 1984;13(3):251–259. doi: 10.1002/jmv.1890130307. [DOI] [PubMed] [Google Scholar]
- Dubin G., Frank I., Friedman H. M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990 Jun;64(6):2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G., Socolof E., Frank I., Friedman H. M. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991 Dec;65(12):7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I., Friedman H. M. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989 Nov;63(11):4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Graziano R. F., Vance B. A., Morganelli P. M., Fanger M. W. Monoclonal antibodies that bind to distinct epitopes on Fc gamma RI are able to trigger receptor function. J Immunol. 1989 Sep 1;143(5):1650–1655. [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Lulitanond V., Johnson D. C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990 Aug;177(2):437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- Hayashida I., Nagafuchi S., Hayashi Y., Kino Y., Mori R., Oda H., Ohtomo N., Tashiro A. Mechanism of antibody-mediated protection against herpes simplex virus infection in athymic nude mice: requirement of Fc portion of antibody. Microbiol Immunol. 1982;26(6):497–509. doi: 10.1111/j.1348-0421.1982.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Lippe P. A., Pauza K. J., Spear P. G., Pereira L., Tevethia S. S. Kinetics of expression of herpes simplex virus type 1-specific glycoprotein species on the surfaces of infected murine, simian, and human cells: flow cytometric analysis. J Virol. 1987 Jan;61(1):104–112. doi: 10.1128/jvi.61.1.104-112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P. J., Myhre E. B., Blomberg J. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J Virol. 1985 Nov;56(2):489–494. doi: 10.1128/jvi.56.2.489-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P. J., Nardella F. A., Sjöquist J., Schröder A. K., Christensen P. Herpes simplex type 1-induced Fc receptor binds to the Cgamma2-Cgamma3 interface region of IgG in the area that binds staphylococcal protein A. Immunology. 1989 Jan;66(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J. Blockade of Fc receptor-mediated binding to U-937 cells by murine monoclonal antibodies directed against a variety of surface antigens. J Immunol. 1983 Jul;131(1):140–147. [PubMed] [Google Scholar]
- Leung-Tack J., Neveu T., Lefroit-Joliy M., Voisin G. A. Alloantibody bipolar bridging; a new mechanism of cell surface activation. Immunol Lett. 1982 Jul;5(1):23–28. doi: 10.1016/0165-2478(82)90086-4. [DOI] [PubMed] [Google Scholar]
- McKendall R. R. IgG-mediated viral clearance in experimental infection with herpes simplex virus type 1: role for neutralization and Fc-dependent functions but not C' cytolysis and C5 chemotaxis. J Infect Dis. 1985 Mar;151(3):464–470. doi: 10.1093/infdis/151.3.464. [DOI] [PubMed] [Google Scholar]
- Neidhardt H., Schröder C. H., Kaerner H. C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J Virol. 1987 Feb;61(2):600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neppert J., Marquard F., Mueller-Eckhardt C. Murine monoclonal antibodies and human alloantisera specific for HLA inhibit monocyte phagocytosis of anti-D-sensitized human red blood cells. Eur J Immunol. 1985 Jun;15(6):559–563. doi: 10.1002/eji.1830150606. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Lausch R. N. Role of Fc fragments in antibody-mediated recovery from ocular and subcutaneous herpes simplex virus infections. Infect Immun. 1981 Jul;33(1):109–114. doi: 10.1128/iai.33.1.109-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein E., Boucheix C., Urso I., Carroll R. C. Fc gamma receptor-mediated interplatelet activation by a monoclonal antibody against beta 2 microglobulin. J Immunol. 1991 Nov 1;147(9):3040–3046. [PubMed] [Google Scholar]
- Schröder A. K., Nardella F. A., Mannik M., Johansson P. J., Christensen P. Identification of the site on IgG Fc for interaction with streptococci of groups A, C and G. Immunology. 1987 Dec;62(4):523–527. [PMC free article] [PubMed] [Google Scholar]
- Tal-Singer R., Seidel-Dugan C., Fries L., Huemer H. P., Eisenberg R. J., Cohen G. H., Friedman H. M. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J Infect Dis. 1991 Oct;164(4):750–753. doi: 10.1093/infdis/164.4.750. [DOI] [PubMed] [Google Scholar]
- Van Kessel K. P., Van Kats-Renaud H. J., Van Strijp J. A., Visser M. R., Verhoef J. Measurement of antibody-mediated binding of human polymorphonuclear leukocytes to HSV-1 infected anchorage fibroblasts. J Immunol Methods. 1986 Apr 3;88(1):101–107. doi: 10.1016/0022-1759(86)90057-8. [DOI] [PubMed] [Google Scholar]
- Van Strijp J. A., Van Kessel K. P., van der Tol M. E., Verhoef J. Complement-mediated phagocytosis of herpes simplex virus by granulocytes. Binding or ingestion. J Clin Invest. 1989 Jul;84(1):107–112. doi: 10.1172/JCI114129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Winkel J. G., Tax W. J., Jacobs C. W., Huizinga T. W., Willems P. H. Cross-linking of both types of IgG Fc receptors, Fc gamma RI and Fc gamma RII, enhances intracellular free Ca2+ in the monocytic cell line U937. Scand J Immunol. 1990 Mar;31(3):315–325. doi: 10.1111/j.1365-3083.1990.tb02774.x. [DOI] [PubMed] [Google Scholar]


