Abstract
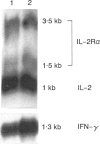
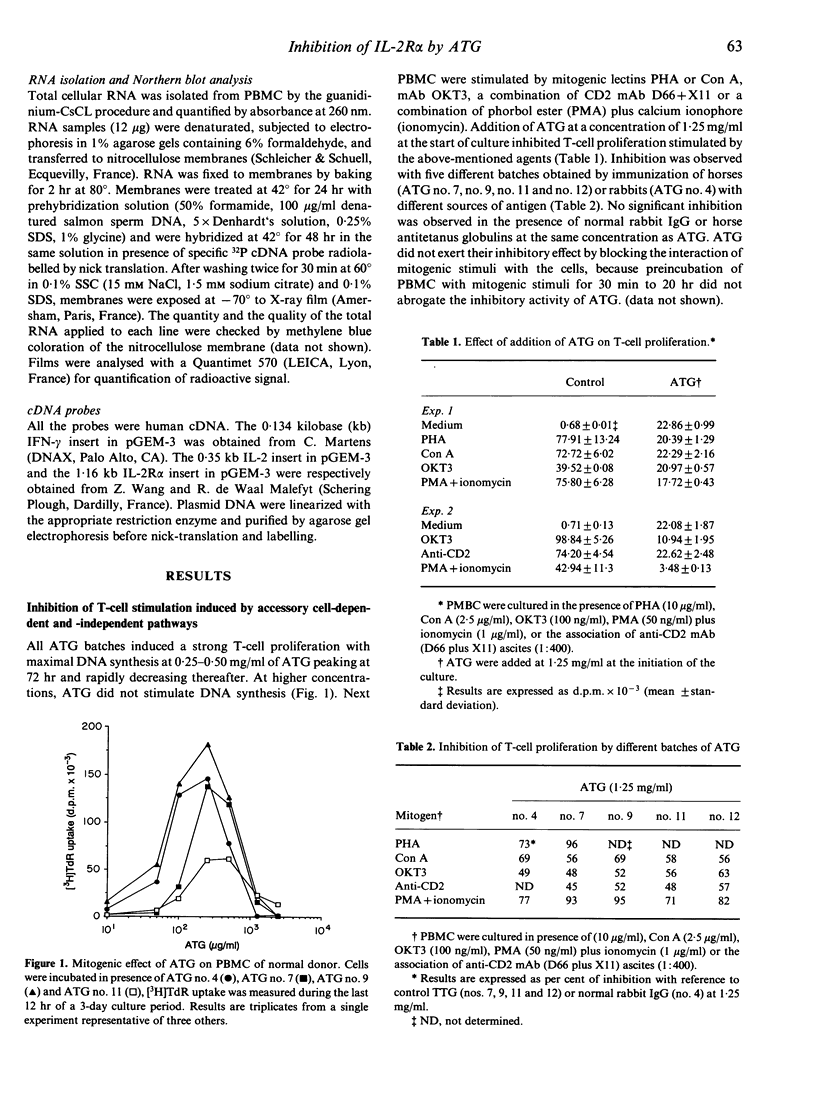
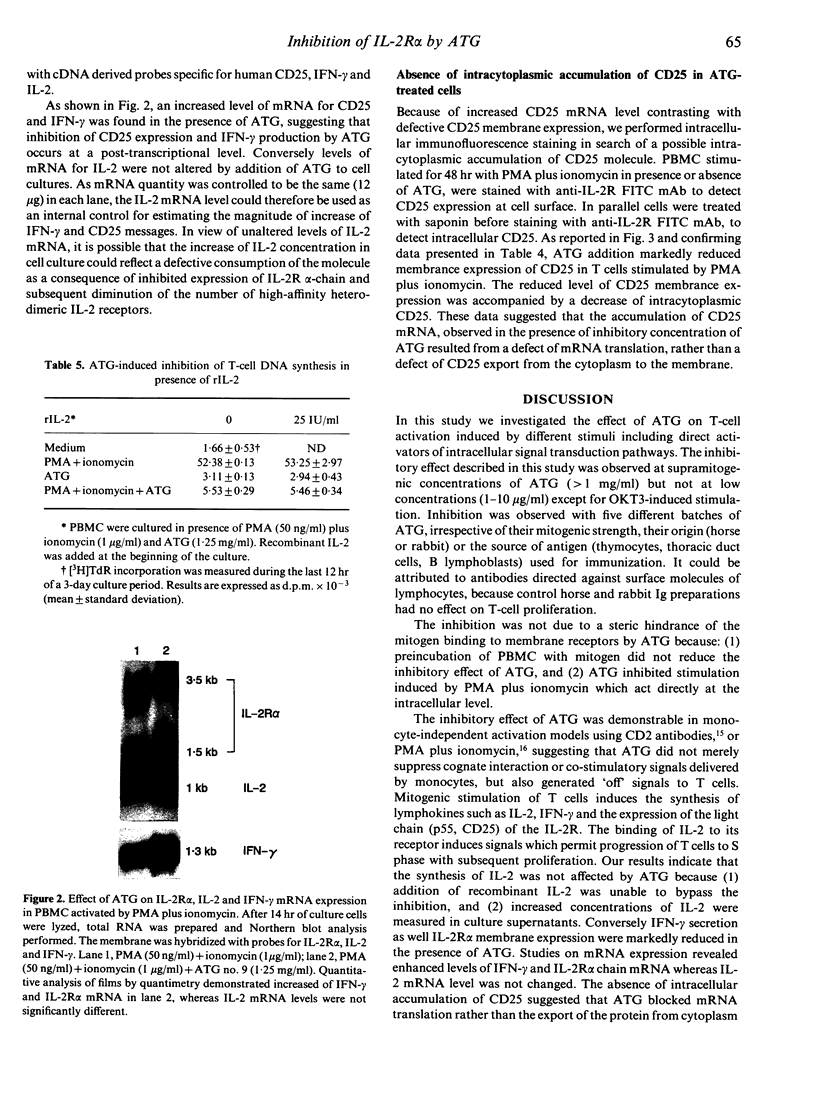
Anti-lymphocyte and anti-thymocyte globulins (ATG) are currently used as immunosuppressive agents in organ transplantation. Their administration in vivo may induce not only lymphocyte depletion but also functional effects which were investigated in the present study. In vitro ATG inhibited T-cell proliferation induced by monocyte-dependent T-cell mitogens, like CD3 antibodies, phytohaemagglutinin (PHA) and concanavalin A (Con A), by monocyte-independent mitogens, like CD2 antibodies, or by protein kinase C activators (phorbol esters) associated with a calcium ionophore. The inhibitory effect of ATG was therefore not solely accounted for by a suppression of co-stimulatory signals delivered by monocytes, but rather implied a direct action on T cells. Addition of recombinant human interleukin-2 (rIL-2) did not overcome the inhibition. Suppression of T-cell proliferation by ATG was characterized by normal RNA synthesis and IL-2 secretion contrasting with markedly reduced expression of the CD25 protein [p55, the alpha-chain of interleukin-2 receptor (IL-2R)] both in cytoplasm and on T-cell membrane, as well as a decreased secretion of interferon-gamma (IFN-gamma). Northern blot analysis revealed increased levels of CD25 and IFN-gamma mRNA, suggesting a post-transcriptional inhibition of these molecules, whereas IL-2 mRNA levels were unchanged. These data demonstrate that inhibition of T-cell proliferation by ATG can be attributed primarily to a post-transcriptional defect of CD25 expression, implying a novel mechanism different from those described with other immunosuppressive agents. Blocking of T-cell proliferation in the late G1 phase of the cell cycle may contribute to the immunosuppressive activity of ATG in prophylactic treatment of allograft rejection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Bonnefoy-Berard N., Flacher M., Revillard J. P. Antiproliferative effect of antilymphocyte globulins on B cells and B-cell lines. Blood. 1992 Apr 15;79(8):2164–2170. [PubMed] [Google Scholar]
- Bonnefoy-Bérard N., Vincent C., Revillard J. P. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation. 1991 Mar;51(3):669–673. doi: 10.1097/00007890-199103000-00024. [DOI] [PubMed] [Google Scholar]
- Brochier J., Revillard J. P. In vitro stimulation or inhibition of lymphocyte activation by antilymphocyte serum. Transplant Proc. 1971 Mar;3(1):788–792. [PubMed] [Google Scholar]
- Carrera A. C., Sanchez-Madrid F., Lopez-Botet M., Bernabeu C., De Landazuri M. O. Involvement of the CD4 molecule in a post-activation event on T cell proliferation. Eur J Immunol. 1987 Feb;17(2):179–186. doi: 10.1002/eji.1830170205. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Bloemmen F. J., Van Wauwe J. P. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J Immunol. 1985 Dec;135(6):3882–3886. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Eiras G., Shimizu Y., van Seventer G. A., Duquesnoy R. J., Zeevi A. Effects of FK 506 and cyclosporine on T-cell activation: integrin-mediated adhesion of T cells, proliferation, and maturation of cytotoxic T cells. Transplant Proc. 1991 Feb;23(1 Pt 2):936–939. [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W. Association of protein kinase C activation with IL 2 receptor expression. J Immunol. 1986 Feb 15;136(4):1266–1273. [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Nolan P., Inaba K., Steinman R. M. The effect of immunosuppressive agents on the induction of nuclear factors that bind to sites on the interleukin 2 promoter. J Exp Med. 1990 Dec 1;172(6):1869–1872. doi: 10.1084/jem.172.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengel H., Allig B., Wagner H., Heeg K. Dissection of signals controlling T cell function and activation: H7, an inhibitor of protein kinase C, blocks induction of primary T cell proliferation by suppressing interleukin (IL)2 receptor expression without affecting IL2 production. Eur J Immunol. 1991 Jul;21(7):1575–1582. doi: 10.1002/eji.1830210702. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for expression of the interleukin 2 receptor. J Immunol. 1985 Mar;134(3):1640–1643. [PubMed] [Google Scholar]
- Najarian J. S., Simmons R. L., Condie R. M., Thompson E. J., Fryd D. S., Howard R. J., Matas A. J., Sutherland D. E., Ferguson R. M., Schmidtke J. R. Seven years' experience with antilymphoblast globulin for renal transplantation from cadaver donors. Ann Surg. 1976 Sep;184(3):352–368. doi: 10.1097/00000658-197609000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. A., Kawamura A., Akselband Y., Peattie D. A., Aldape R. A., Harding M. W. Effect of immunosuppressive drugs on cytokine gene transcription studied by message amplification phenotyping (MAPPing) polymerase chain reaction. Transplant Proc. 1991 Dec;23(6):2867–2869. [PubMed] [Google Scholar]
- Polke C. R., Lowenthal J. W., Roth S. A., Rohwer P., MacDonald H. R., Kalden J. R. Phorbol ester enhances both interleukin 2 receptor expression and immunoglobulin secretion in human Epstein-Barr virus-immortalized B cells. Eur J Immunol. 1986 Feb;16(2):146–150. doi: 10.1002/eji.1830160207. [DOI] [PubMed] [Google Scholar]
- Revillard J. P., Brochier J., Traeger J., Balner H. In vitro assay of suppressive properties of antilymphocyte sera on lymphocyte stimulation. Transplantation. 1970 Jun;9(6):592–597. doi: 10.1097/00007890-197006000-00017. [DOI] [PubMed] [Google Scholar]
- Sakane T., Takada S. Possible involvement of the CD4 molecule in a late activation event on CD4+ T cell proliferation in the human autologous mixed lymphocyte reaction. Clin Exp Immunol. 1989 Feb;75(2):269–274. [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Turco M. C., De Felice M., Corbo L., Morrone G., Mertelsmann R., Ferrone S., Venuta S. Regulatory role of a monomorphic determinant of HLA Class I antigens in T cell proliferation. J Immunol. 1985 Oct;135(4):2268–2273. [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]