Abstract
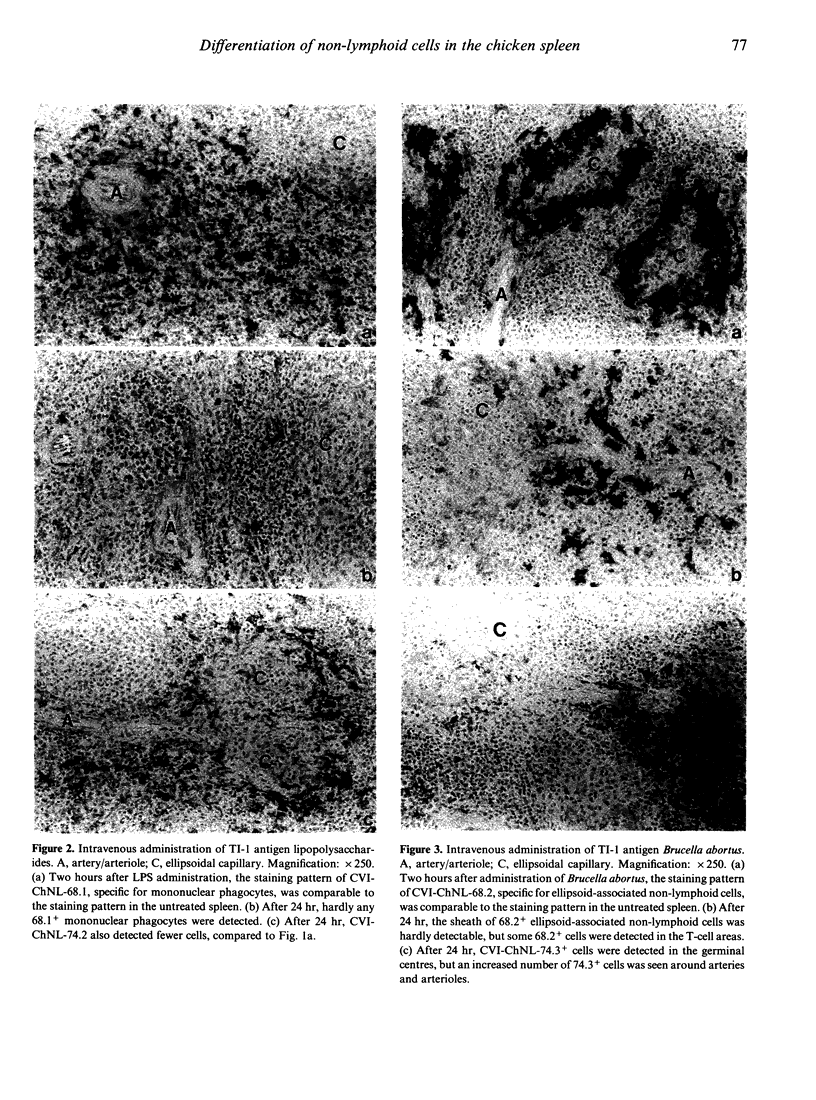
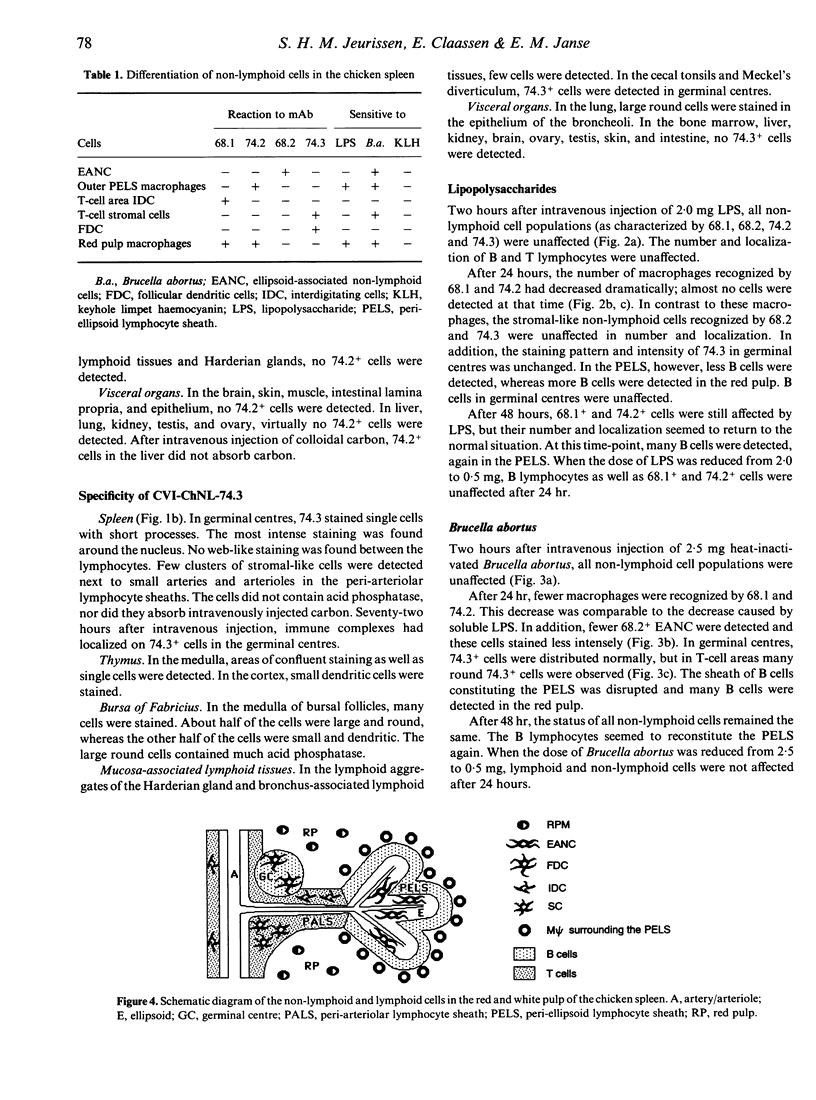
The phenotypes and functions of various populations of non-lymphoid cells in the chicken spleen were investigated with monoclonal antibodies and after in vivo administration of antigens. Monoclonal antibody CVI-ChNL-68.1 was used to detect red pulp macrophages, interdigitating cells, and monocytes, whereas CVI-ChNL-68.2 was used to detect ellipsoid-associated non-lymphoid cells (EANC). Two new monoclonal antibodies were developed: CVI-ChNL-74.2, which specifically recognized red pulp macrophages and a ring of macrophages surrounding the peri-ellipsoid lymphocyte sheath; and CVI-ChNL-74.3, which specifically recognized follicular dendritic cells (FDC) in germinal centres and small clusters of stromal cells in T-cell areas. After intravenous injection of lipopolysaccharide (LPS), the number of 68.1+ and 74.2+ macrophages decreased dramatically, whereas 68.2+ EANC and 74.3+ FDC were unaffected. After intravenous injection of heat-inactivated Brucella abortus, the numbers of both macrophages and EANC decreased. In contrast, a significant increase of 74.3+ cells was observed in the T-cell areas outside the germinal centres. As expected, intravenous injection of non-mitogenic antigens, such as keyhole limpet haemocyanin and Ficoll, and carrageenan did not affect the non-lymphoid cell populations. At least six subpopulations of non-lymphoid cells in the chicken spleen can now be discriminated with monoclonal antibodies. Our results show that mononuclear phagocytes are sensitive for mitogenic stimulators such as LPS and Brucella abortus. In contrast, stromal non-lymphoid cells are only sensitive to the particulate mitogen Brucella abortus. We conclude that the complex formed by ellipsoid cells, the peri-ellipsoid B-cell sheath, and the surrounding macrophages, is the functional equivalent of the mammalian marginal zone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschheim L., Raffel S. The immunodepressant effect of carrageenin. J Reticuloendothel Soc. 1972 Mar;11(3):253–262. [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-trinitrophenyl antibody-forming cells in the murine spleen. Eur J Immunol. 1986 Mar;16(3):271–276. doi: 10.1002/eji.1830160311. [DOI] [PubMed] [Google Scholar]
- Goud S. N., Muthusamy N., Subbarao B. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988 May 1;140(9):2925–2930. [PubMed] [Google Scholar]
- Humphrey J. H., Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981 Mar;11(3):221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- Janse E. M., Jeurissen S. H. Ontogeny and function of two non-lymphoid cell populations in the chicken embryo. Immunobiology. 1991 Aug;182(5):472–481. doi: 10.1016/s0171-2985(11)80211-1. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Janse E. M., Ekino S., Nieuwenhuis P., Koch G., De Boer G. F. Monoclonal antibodies as probes for defining cellular subsets in the bone marrow, thymus, bursa of fabricius, and spleen of the chicken. Vet Immunol Immunopathol. 1988 Oct;19(3-4):225–238. doi: 10.1016/0165-2427(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Janse E. M., Koch G., de Boer G. F. The monoclonal antibody CVI-ChNL-68.1 recognizes cells of the monocyte-macrophage lineage in chickens. Dev Comp Immunol. 1988 Fall;12(4):855–864. doi: 10.1016/0145-305x(88)90059-6. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Janse E. M., Kok G. L., De Boer G. F. Distribution and function of non-lymphoid cells positive for monoclonal antibody CVI-ChNL-68.2 in healthy chickens and those infected with Marek's disease virus. Vet Immunol Immunopathol. 1989 Sep;22(2):123–133. doi: 10.1016/0165-2427(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H. Structure and function of the chicken spleen. Res Immunol. 1991 May;142(4):352–355. doi: 10.1016/0923-2494(91)90090-6. [DOI] [PubMed] [Google Scholar]
- Murthy K. K., Ragland W. L. Modification of humoral immune response in chickens following treatment with carrageenan. Vet Immunol Immunopathol. 1984 Oct;7(3-4):347–357. doi: 10.1016/0165-2427(84)90092-8. [DOI] [PubMed] [Google Scholar]
- Noteborn M. H., de Boer G. F., van Roozelaar D. J., Karreman C., Kranenburg O., Vos J. G., Jeurissen S. H., Hoeben R. C., Zantema A., Koch G. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol. 1991 Jun;65(6):3131–3139. doi: 10.1128/jvi.65.6.3131-3139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh I., Glick B., Taylor R. L., Jr Effect of soluble antigen on the ellipsoid-associated cells of the chicken's spleen. J Leukoc Biol. 1984 May;35(5):501–510. doi: 10.1002/jlb.35.5.501. [DOI] [PubMed] [Google Scholar]
- Rademakers L. H. Follicular dendritic cells in germinal centre development. Res Immunol. 1991 Mar-Apr;142(3):257–260. doi: 10.1016/0923-2494(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Terashima K., Dobashi M., Maeda K., Imai Y. Cellular components involved in the germinal centre reaction. Res Immunol. 1991 Mar-Apr;142(3):263–268. doi: 10.1016/0923-2494(91)90073-r. [DOI] [PubMed] [Google Scholar]
- White R. G., French V. I., Stark J. M. A study of the localisation of a protein antigen in the chicken spleen and its relation to the formation of germinal centres. J Med Microbiol. 1970 Feb;3(1):65–83. doi: 10.1099/00222615-3-1-65. [DOI] [PubMed] [Google Scholar]
- White R. G., Henderson D. C., Eslami M. B., Neilsen K. H. Localization of a protein antigen in the chicken spleen. Effect of various manipulative procedures on the morphogenesis of the germinal centre. Immunology. 1975 Jan;28(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N. Antigen processing and presentation in vivo: the microenvironment as a crucial factor. Immunol Today. 1990 Dec;11(12):436–439. doi: 10.1016/0167-5699(90)90171-5. [DOI] [PubMed] [Google Scholar]
- van den Eertwegh A. J., Fasbender M. J., Schellekens M. M., van Oudenaren A., Boersma W. J., Claassen E. In vivo kinetics and characterization of IFN-gamma-producing cells during a thymus-independent immune response. J Immunol. 1991 Jul 15;147(2):439–446. [PubMed] [Google Scholar]