Abstract
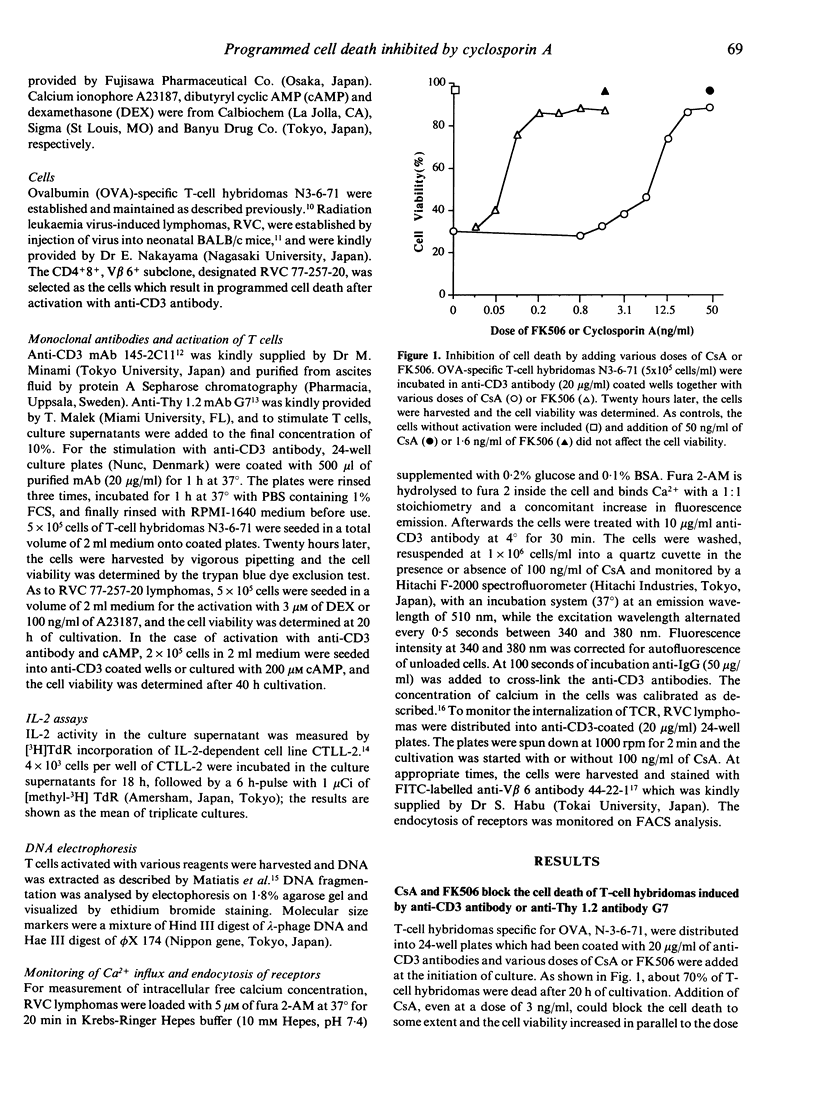
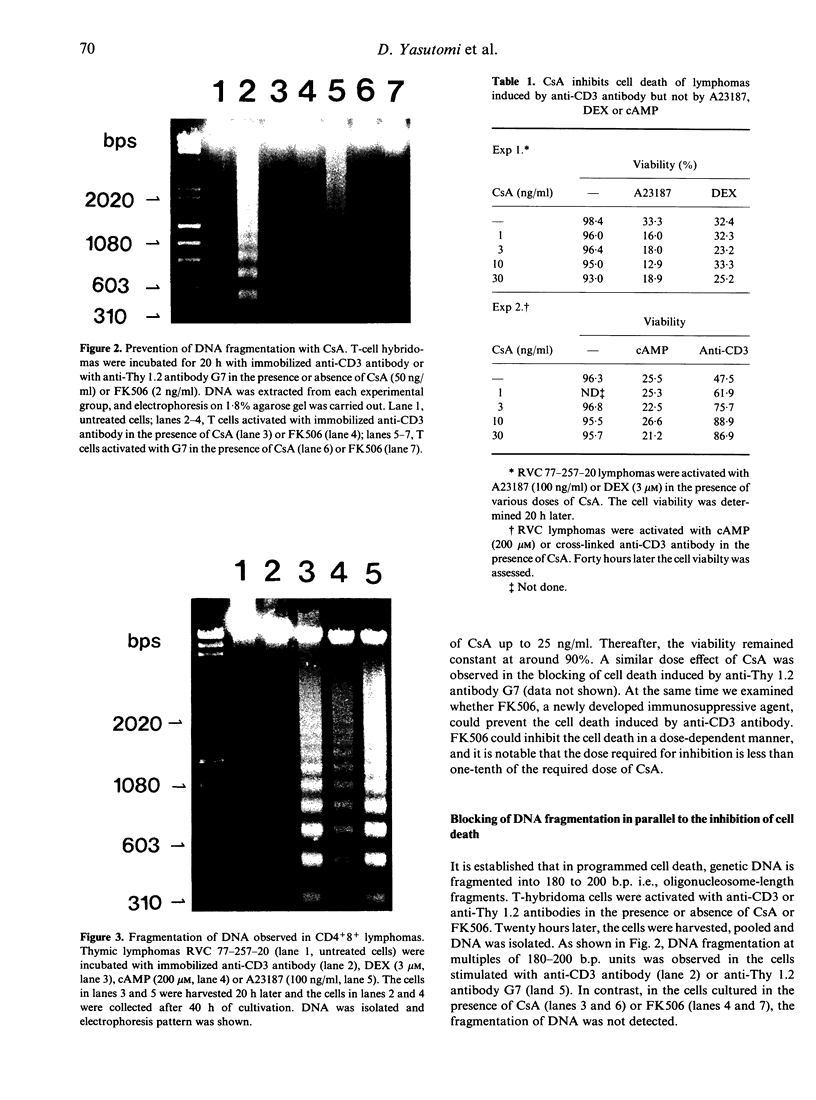
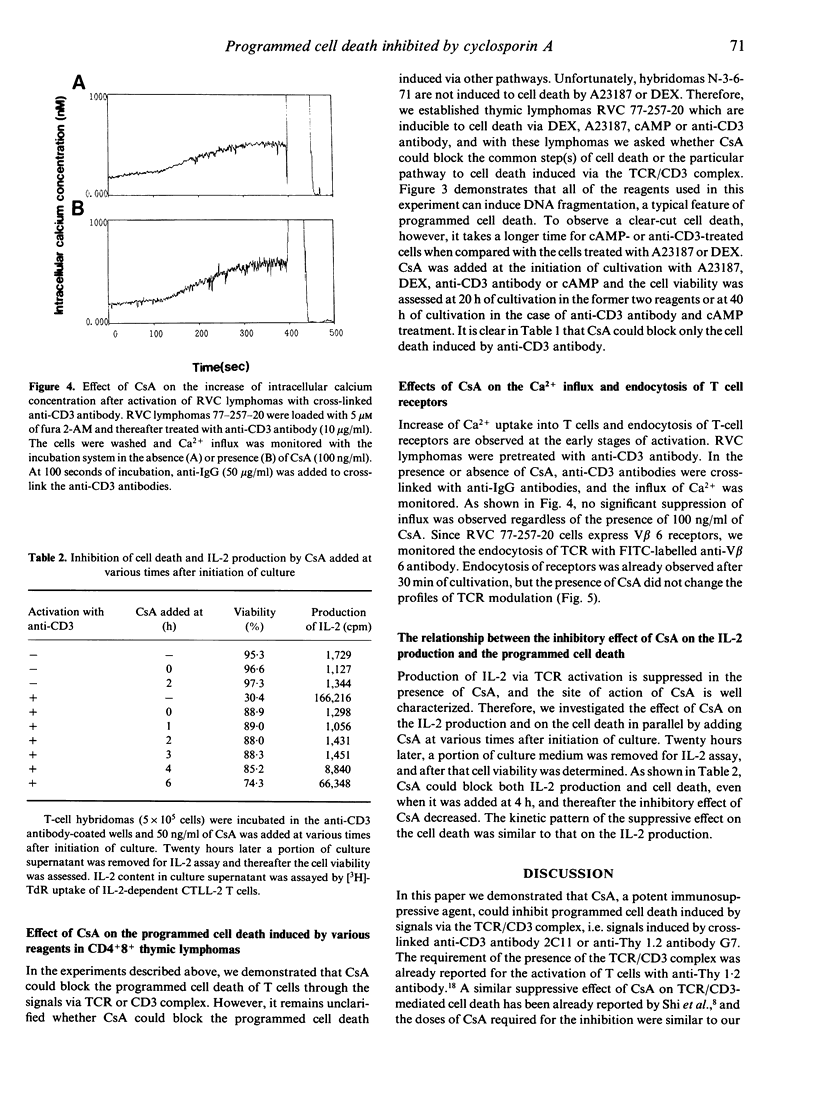
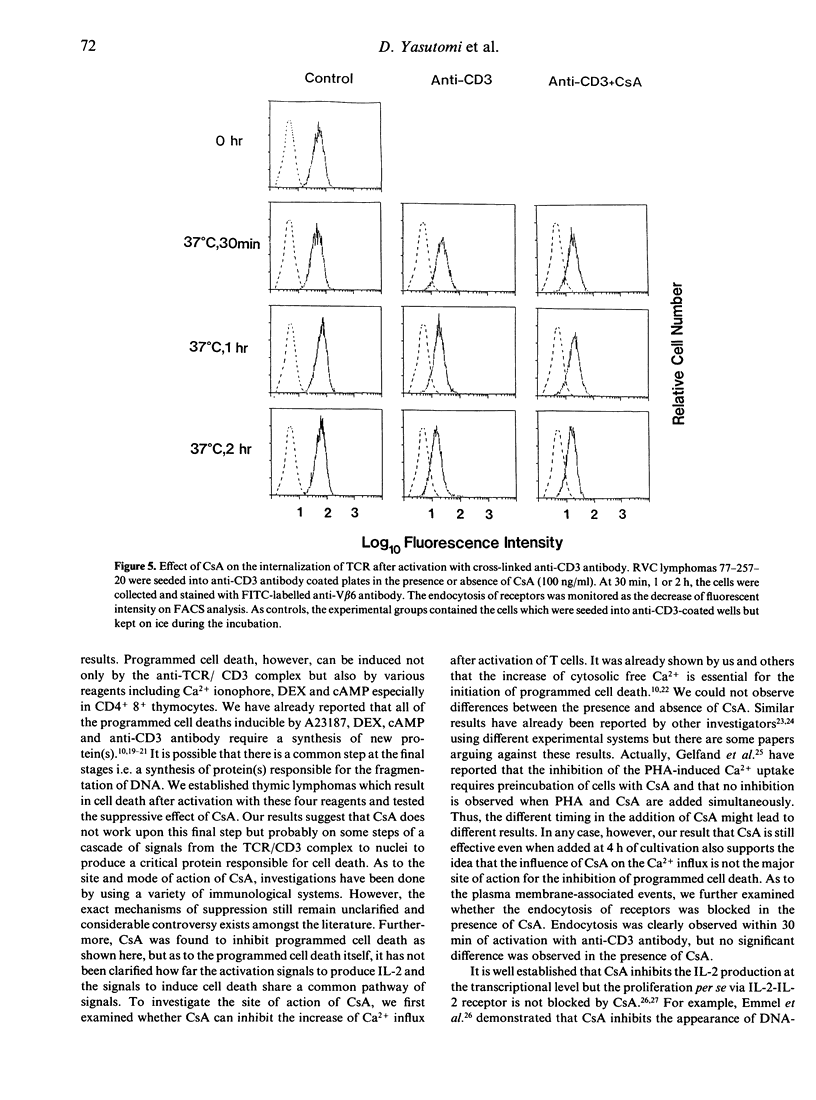
Cyclosporin A (CsA) is reported to inhibit programmed cell death. We confirmed this by using T-cell hybridomas which are inducible to programmed cell death by activation with immobilized anti-CD3 antibody or with anti-Thy 1.2 antibody. Cell death and DNA fragmentation, characteristic features of programmed cell death, were almost completely blocked by CsA or FK506. To investigate whether CsA inhibits only the cell death through the signals via the TCR/CD3 complex or all of the programmed cell death induced by various reagents, we further established CD4+8+ thymic lymphomas which result in programmed cell death after activation with calcium ionophore, dexamethasone, cyclic AMP or anti-CD3 antibody. It was revealed that CsA could block only the cell death mediated by the TCR/CD3 complex. For the clarification of the site of action of CsA, Ca2+ influx and endocytosis of receptors after stimulation with anti-CD3 antibody were monitored in the presence of CsA, and no significant effects of CsA were observed. Furthermore, prevention of cell death was examined by adding CsA at various periods of time after initiation of culture. CsA was found to exert its effect even when added after 4 h of cultivation, and the kinetic pattern of suppression was similar to that of the suppressive effect on IL-2 production. These observations indicate that in the events of programmed cell death, the major site of action of CsA will not be the inhibition of the immediate membrane events after activation of the TCR/CD3 complex but rather the interference in the function of molecules that transmit signals between membrane events and the activation of genes in the nucleus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Cheney R., Kanagawa O., Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988 Nov 10;336(6195):176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Mills G. B. The cyclosporins inhibit lymphocyte activation at more than one site. J Immunol. 1987 Feb 15;138(4):1115–1120. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glazier A., Tutschka P. J., Farmer E. R., Santos G. W. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983 Jul 1;158(1):1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter K. C., Germain R. N., Kroczek R. A., Saito T., Yokoyama W. M., Chan C., Weiss A., Shevach E. M. Thy-1-mediated T-cell activation requires co-expression of CD3/Ti complex. Nature. 1987 Apr 2;326(6112):505–507. doi: 10.1038/326505a0. [DOI] [PubMed] [Google Scholar]
- Gunter K. C., Malek T. R., Shevach E. M. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984 Mar 1;159(3):716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Hudnall S. D. Cyclosporin A renders target cells resistant to immune cytolysis. Eur J Immunol. 1991 Jan;21(1):221–226. doi: 10.1002/eji.1830210133. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H., Pardoll D. M. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988 Sep 23;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- Kizaki H., Suzuki K., Tadakuma T., Ishimura Y. Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J Biol Chem. 1990 Mar 25;265(9):5280–5284. [PubMed] [Google Scholar]
- Kizaki H., Tadakuma T., Odaka C., Muramatsu J., Ishimura Y. Activation of a suicide process of thymocytes through DNA fragmentation by calcium ionophores and phorbol esters. J Immunol. 1989 Sep 15;143(6):1790–1794. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Metcalfe S. Cyclosporine does not prevent cytoplasmic calcium changes associated with lymphocyte activation. Transplantation. 1984 Aug;38(2):161–164. doi: 10.1097/00007890-198408000-00014. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Uenaka A., Stockert E., Obata Y. Detection of a unique antigen on radiation leukemia virus-induced leukemia B6RV2. Cancer Res. 1984 Nov;44(11):5138–5144. [PubMed] [Google Scholar]
- Odaka C., Kizaki H., Tadakuma T. T cell receptor-mediated DNA fragmentation and cell death in T cell hybridomas. J Immunol. 1990 Mar 15;144(6):2096–2101. [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. K., Standaert R. F., Galat A., Nakatsuka M., Schreiber S. L. Inhibition of FKBP rotamase activity by immunosuppressant FK506: twisted amide surrogate. Science. 1990 May 18;248(4957):863–866. doi: 10.1126/science.1693013. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporin A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988 Apr 1;167(4):1479–1485. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Suzuki G., Kawase Y., Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987 Sep 15;139(6):1797–1803. [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Siekierka J. J., Staruch M. J., Hung S. H., Sigal N. H. FK-506, a potent novel immunosuppressive agent, binds to a cytosolic protein which is distinct from the cyclosporin A-binding protein, cyclophilin. J Immunol. 1989 Sep 1;143(5):1580–1583. [PubMed] [Google Scholar]
- Sorokin R., Kimura H., Schroder K., Wilson D. H., Wilson D. B. Cyclosporine-induced autoimmunity. Conditions for expressing disease, requirement for intact thymus, and potency estimates of autoimmune lymphocytes in drug-treated rats. J Exp Med. 1986 Nov 1;164(5):1615–1625. doi: 10.1084/jem.164.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Stiller C. R., Dupré J., Gent M., Jenner M. R., Keown P. A., Laupacis A., Martell R., Rodger N. W., von Graffenried B., Wolfe B. M. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984 Mar 30;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Tadakuma T., Kizaki H., Odaka C., Kubota R., Ishimura Y., Yagita H., Okumura K. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur J Immunol. 1990 Apr;20(4):779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Weiss A., Imboden J., Kamin-Lewis R., Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985 Mar;134(3):1599–1603. [PubMed] [Google Scholar]