Abstract
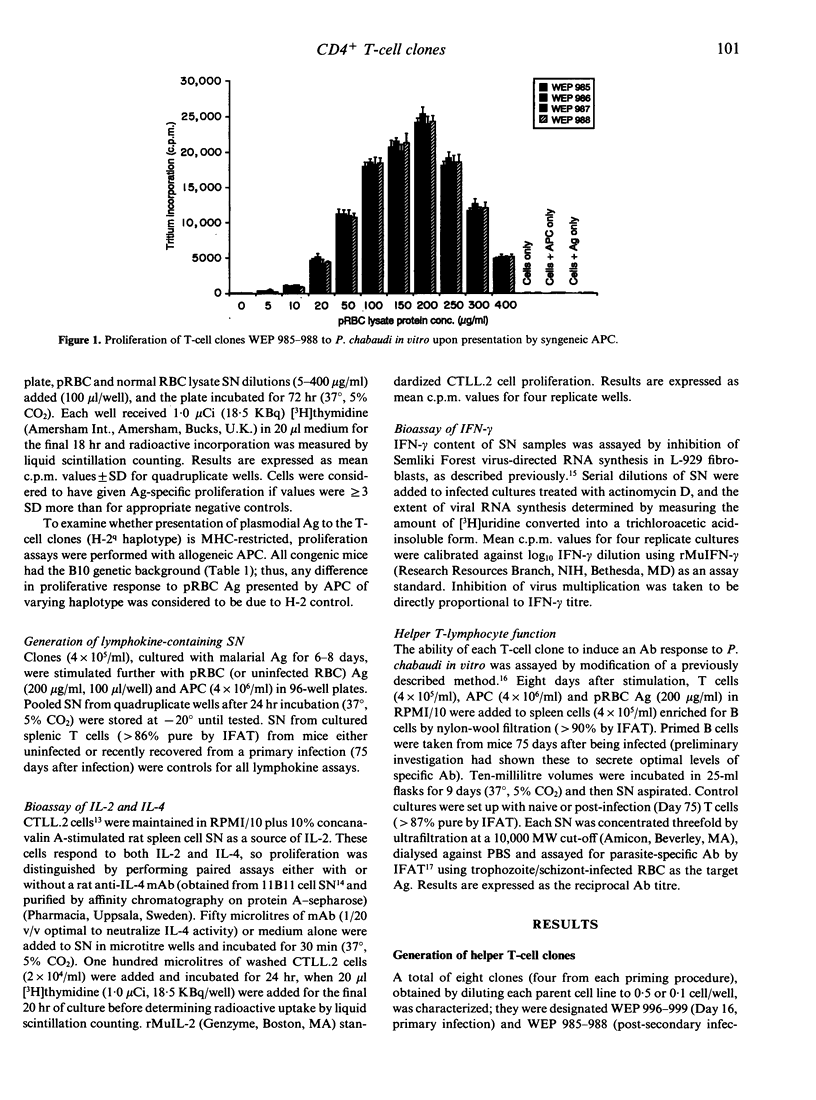
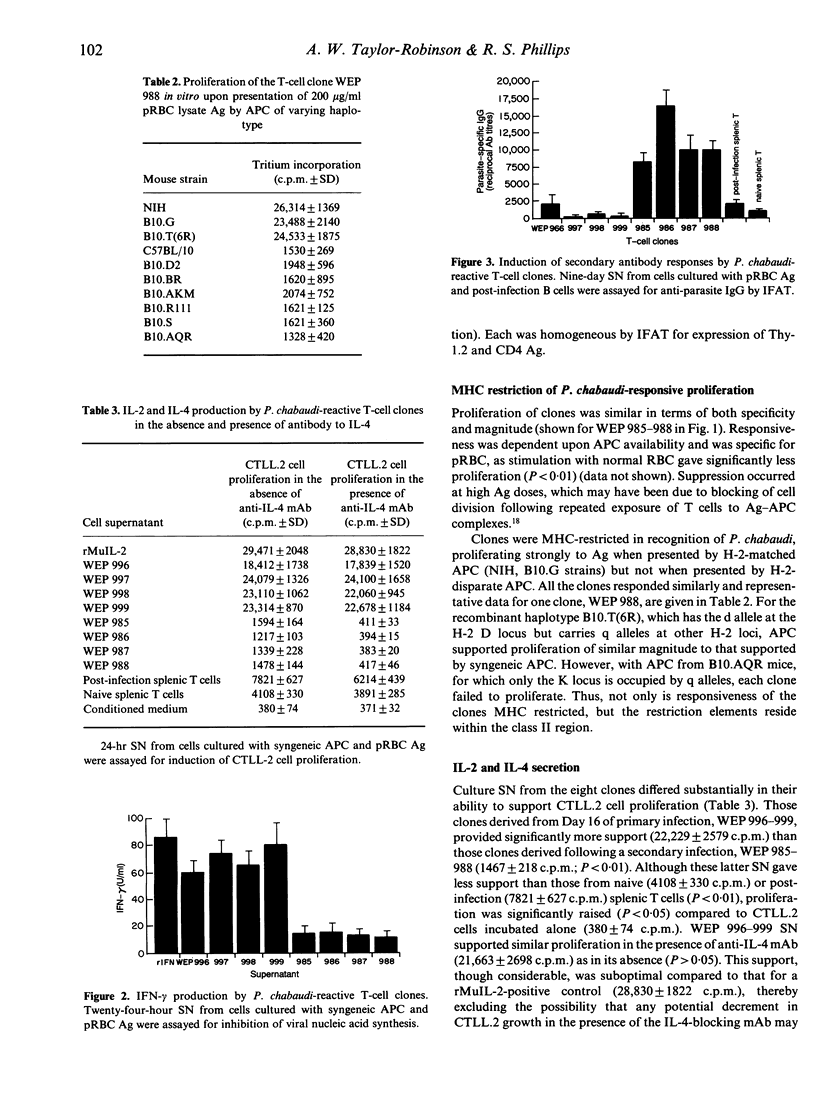
Protective immunity to asexual malaria parasites appears to be mediated predominantly by the CD4+ subset of T lymphocytes. To examine the role of this T-cell population in the immune response to the murine malaria parasite Plasmodium chabaudi, CD4+ clones derived from infected mice were raised and propagated in vitro. Analysis of the reactivity of clones responsive to parasite antigen demonstrated that the CD4+ cell response is heterogeneous and is consistent with the idea of two functionally distinct CD4+ subsets. Those populations derived early during primary infection secreted interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) upon antigenic stimulation in vitro, i.e. they had a cytokine repertoire typical of the delayed-type inflammatory T-helper 1 (Th1) CD4+ subset. In contrast, cells taken after clearance of a secondary infection produced IL-4 and acted as effective helper cells for anti-malarial antibody (Ab) synthesis in vitro, and thereby had the characteristics of Th2 cells. The appearance in vivo of Th1 and then Th2 clones specific for P. chabaudi-parasitized erythrocytes (pRBC) supports the proposal from limiting culture analyses that for this malaria parasite resolution of primary parasitaemia is predominantly through the action of cytokines rather than Ab, and that final clearance requires helper cells and specific immunoglobulin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. T., Giron D. J. Rapid sensitive assay for interferons based on the inhibition of MM virus nucleic acid synthesis. Appl Microbiol. 1970 Sep;20(3):317–322. doi: 10.1128/am.20.3.317-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Falanga P. B., D'Imperio Lima M. R., Coutinho A., Pereira da Silva L. Isotypic pattern of the polyclonal B cell response during primary infection by Plasmodium chabaudi and in immune-protected mice. Eur J Immunol. 1987 May;17(5):599–603. doi: 10.1002/eji.1830170504. [DOI] [PubMed] [Google Scholar]
- Fathman C. G., Kimoto M. Studies Utilizing murine T cell clones: Ir genes, Ia antigens and MLR stimulating determinants. Immunol Rev. 1981;54:57–79. doi: 10.1111/j.1600-065x.1981.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Evans C. B., Asofsky R., Taylor D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1984 Sep;87(2):452–461. doi: 10.1016/0008-8749(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Gillard S., Simon B., Slade S., Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1(4):416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Meding S. J., Eichmann K., Gillard S. S. The response of CD4+ T cells to Plasmodium chabaudi chabaudi. Immunol Rev. 1989 Dec;112:71–94. doi: 10.1111/j.1600-065x.1989.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Simon B. Limiting dilution analysis of the T cell response to Plasmodium chabaudi chabaudi in mice. Parasite Immunol. 1989 Sep;11(5):545–559. doi: 10.1111/j.1365-3024.1989.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989 Nov;5(11):362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V., Sherman I. W. Plasmodium chabaudi: humoral and cell-mediated responses of immunized mice. Exp Parasitol. 1980 Jun;49(3):442–454. doi: 10.1016/0014-4894(80)90078-8. [DOI] [PubMed] [Google Scholar]
- McLean S. A., Pearson C. D., Phillips R. S. Plasmodium chabaudi: relationship between the occurrence of recrudescent parasitaemias in mice and the effective levels of acquired immunity. Exp Parasitol. 1982 Oct;54(2):213–221. doi: 10.1016/0014-4894(82)90129-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Pearson C. D., McLean S. A., Tetley K., Phillips R. S. Induction of secondary antibody responses to Plasmodium chabaudi in vitro. Clin Exp Immunol. 1983 Apr;52(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Mond J. J., Ohara J., Paul W. E. Interferon-gamma inhibits the action of B cell stimulatory factor (BSF)-1 on resting B cells. J Immunol. 1986 Sep 1;137(5):1573–1576. [PubMed] [Google Scholar]
- Riedlinger J., Grencis R. K., Wakelin D. Antigen-specific T-cell lines transfer protective immunity against Trichinella spiralis in vivo. Immunology. 1986 May;58(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Belosevic M., van der Meide P. H., Podoba J. E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990 Oct;58(10):3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G., Kawase Y., Koyasu S., Yahara I., Kobayashi Y., Schwartz R. H. Antigen-induced suppression of the proliferative response of T cell clones. J Immunol. 1988 Mar 1;140(5):1359–1365. [PubMed] [Google Scholar]
- Süss G., Eichmann K., Kury E., Linke A., Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988 Dec;56(12):3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Opposing effects of glucocorticoids on interferon-gamma-induced murine macrophage Fc receptor and Ia antigen expression. J Immunol. 1985 Apr;134(4):2462–2469. [PubMed] [Google Scholar]


