Abstract
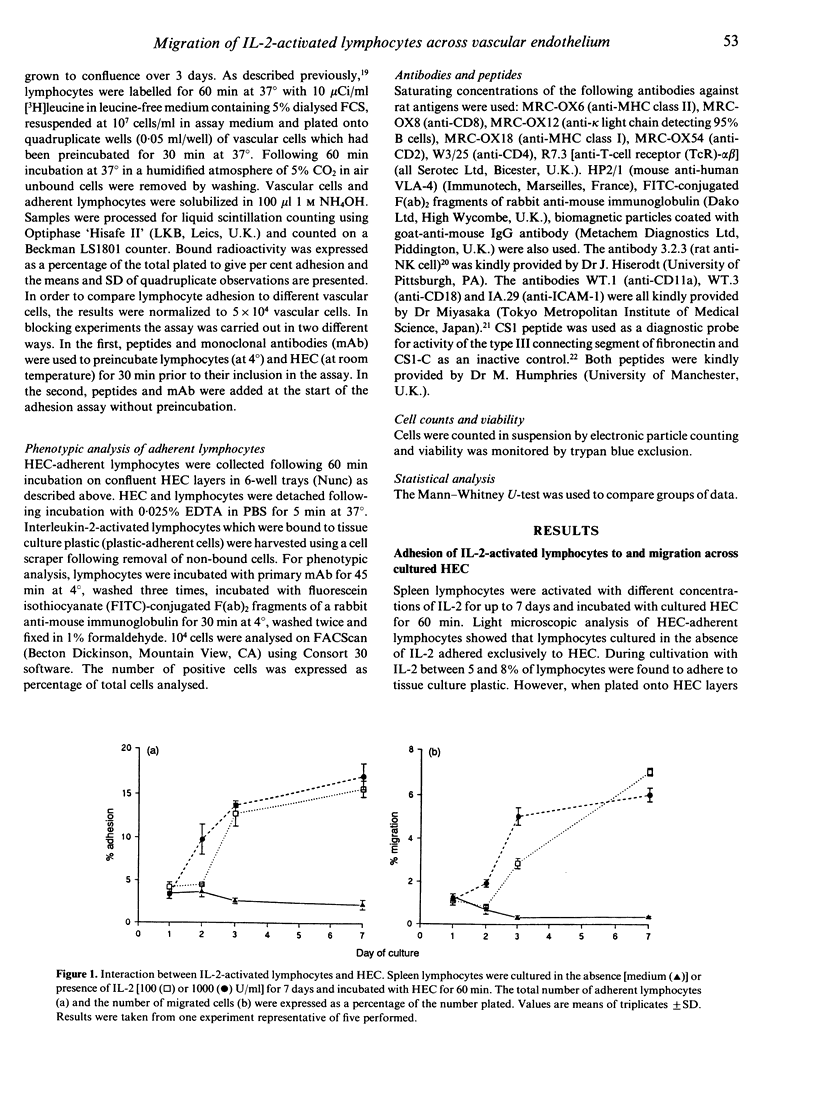
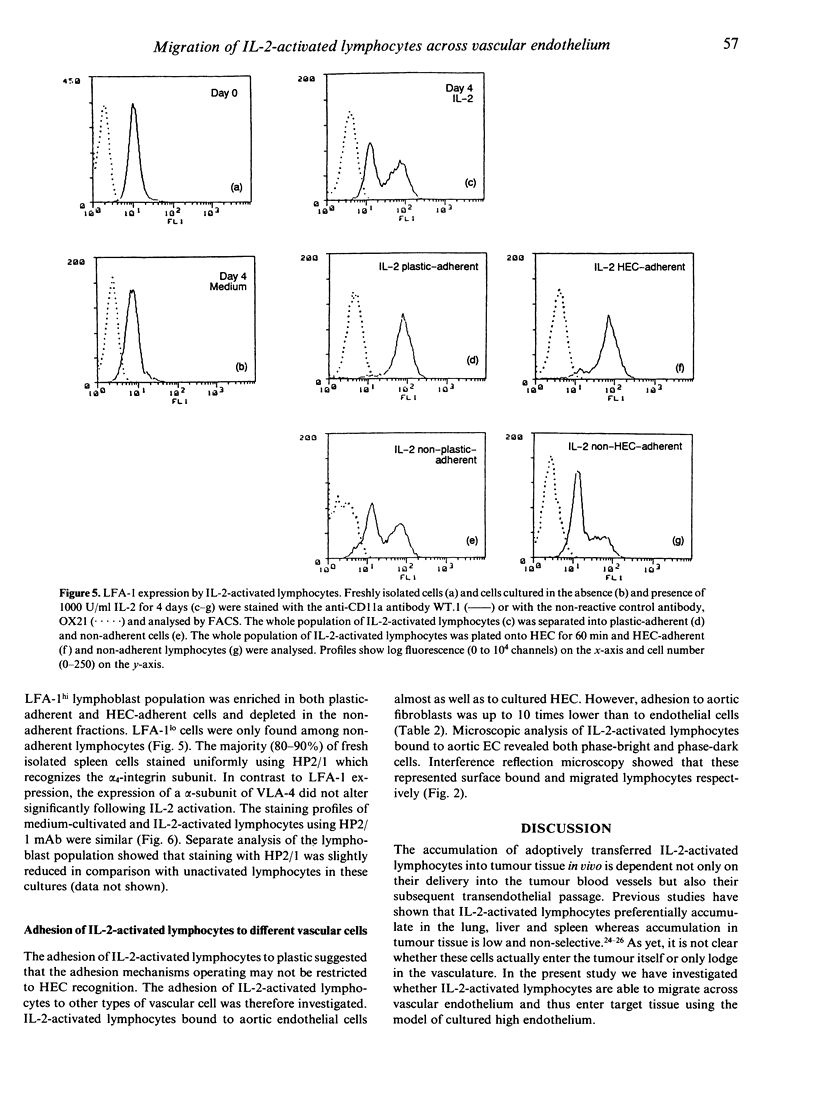
A prerequisite for the successful immunotherapy of solid tumours with interleukin-2 (IL-2)-activated lymphocytes is their ability to home to the tumour tissue. Lymphocyte homing is a complex process which is known to involve at least two independently regulated events: adhesion to the luminal surface of vascular endothelium and the subsequent transendothelial migration of lymphocytes. In this study we have used an in vitro model of lymphocyte homing which employs specialized high endothelium to ask whether IL-2-activated lymphocytes are able to migrate across vascular endothelium in order to leave the blood vessel. Both the adhesion of IL-2-activated cells and their migration across monolayers of cultured high endothelial cells (HEC) were increased in comparison with non-activated lymphocytes. The adhesion of IL-2-activated lymphocytes was mediated by lymphocyte function-associated antigen-1 (LFA-1) and a very late activation antigen-4 (VLA-4)-related pathway. LFA-1-dependent adhesion was mediated by ligands on HEC other than the intercellular adhesion molecule-1 (ICAM-1) and the VLA-4-related pathway was mediated by ligands other than the CS1 domain of fibronectin. HEC-adherent lymphocytes were enriched in natural killer (NK) cells and CD8+ T cells which are known to be the tumour-cytotoxic cells in IL-2-activated lymphocytes. However, there was no evidence of cytotoxicity towards the endothelial layer using a syngeneic model. The interaction of IL-2-activated lymphocytes and endothelial cells was not specific for high endothelium since equal numbers of activated lymphocytes bound to and migrated across aortic endothelium. The inability of IL-2-activated lymphocytes to discriminate between high endothelium and non-specialized 'flat' endothelium could be responsible for the widespread dissemination of the cells throughout the body following their adoptive transfer and the unwanted side-effects at non-involved sites.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Humphries M. J. Use of synthetic peptides to probe lymphocyte--high endothelial cell interactions. Lymphocytes recognize a ligand on the endothelial surface which contains the CS1 adhesion motif. Int Immunol. 1990;2(10):921–928. doi: 10.1093/intimm/2.10.921. [DOI] [PubMed] [Google Scholar]
- Ager A. Isolation and culture of high endothelial cells from rat lymph nodes. J Cell Sci. 1987 Feb;87(Pt 1):133–144. doi: 10.1242/jcs.87.1.133. [DOI] [PubMed] [Google Scholar]
- Ager A., Mistry S. Interaction between lymphocytes and cultured high endothelial cells: an in vitro model of lymphocyte migration across high endothelial venule endothelium. Eur J Immunol. 1988 Aug;18(8):1265–1274. doi: 10.1002/eji.1830180818. [DOI] [PubMed] [Google Scholar]
- Allavena P., Paganin C., Martin-Padura I., Peri G., Gaboli M., Dejana E., Marchisio P. C., Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991 Feb 1;173(2):439–448. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse P., Herberman R. B., Nannmark U., Johansson B. R., Hokland M., Wasserman K., Goldfarb R. H. Accumulation of adoptively transferred adherent, lymphokine-activated killer cells in murine metastases. J Exp Med. 1991 Aug 1;174(2):479–488. doi: 10.1084/jem.174.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers W. H., Vujanovic N. L., DeLeo A. B., Olszowy M. W., Herberman R. B., Hiserodt J. C. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989 Apr 1;169(4):1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Bender J. R., Bradley E. C. Interleukin 2-activated human lymphocytes exhibit enhanced adhesion to normal vascular endothelial cells and cause their lysis. J Immunol. 1987 Mar 15;138(6):1779–1785. [PubMed] [Google Scholar]
- Dissen E., Vaage J. T., Taskén K., Jahnsen T., Rolstad B., Fossum S. Alloreactive lymphokine-activated killer cells from athymic nude rats do not express CD3-associated alpha/beta or gamma/delta T cell receptors. Int Immunol. 1990;2(5):453–460. doi: 10.1093/intimm/2.5.453. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Ford W. L. Functional and morphological changes in post-capillary venules in relation to lymphocytic infiltration into BCG-induced granulomata in rat skin. J Pathol. 1985 Sep;147(1):1–12. doi: 10.1002/path.1711470102. [DOI] [PubMed] [Google Scholar]
- Freemont A. J. The small blood vessels in areas of lymphocytic infiltration around malignant neoplasms. Br J Cancer. 1982 Aug;46(2):283–288. doi: 10.1038/bjc.1982.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Thiele H. G. Molecules and regulation in lymphocyte migration. Immunol Rev. 1989 Apr;108:19–44. doi: 10.1111/j.1600-065x.1989.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Weissman I. L. Peyer's patch-specific lymphocyte homing receptors consist of a VLA-4-like alpha chain associated with either of two integrin beta chains, one of which is novel. EMBO J. 1989 Jun;8(6):1735–1741. doi: 10.1002/j.1460-2075.1989.tb03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland G. W., Dopping-Hepenstal P. J., Jordan P. W., O'Neill C. H. Limitation of substratum size alters cytoskeletal organization and behaviour of Swiss 3T3 fibroblasts. Cell Biol Int Rep. 1989 Sep;13(9):781–790. doi: 10.1016/0309-1651(89)90055-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Ding A., Evans E. L., Krensky A. M., Clayberger C., Phillips J. H. Expression of Leu-19 (NKH-1) antigen on IL 2-dependent cytotoxic and non-cytotoxic T cell lines. J Immunol. 1987 Apr 1;138(7):2019–2023. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Lotze M. T., Line B. R., Mathisen D. J., Rosenberg S. A. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J Immunol. 1980 Oct;125(4):1487–1493. [PubMed] [Google Scholar]
- May M. J., Ager A. ICAM-1-independent lymphocyte transmigration across high endothelium: differential up-regulation by interferon gamma, tumor necrosis factor-alpha and interleukin 1 beta. Eur J Immunol. 1992 Jan;22(1):219–226. doi: 10.1002/eji.1830220132. [DOI] [PubMed] [Google Scholar]
- Melder R. J., Whiteside T. L., Vujanovic N. L., Hiserodt J. C., Herberman R. B. A new approach to generating antitumor effectors for adoptive immunotherapy using human adherent lymphokine-activated killer cells. Cancer Res. 1988 Jun 15;48(12):3461–3469. [PubMed] [Google Scholar]
- Miltenburg A. M., Meijer-Paape M. E., Daha M. R., Paul L. C. Endothelial cell lysis induced by lymphokine-activated human peripheral blood mononuclear cells. Eur J Immunol. 1987 Sep;17(9):1383–1386. doi: 10.1002/eji.1830170926. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Humphries M. J. Identification of a novel recognition sequence for the integrin alpha 4 beta 1 in the COOH-terminal heparin-binding domain of fibronectin. EMBO J. 1991 Dec;10(13):4089–4095. doi: 10.1002/j.1460-2075.1991.tb04985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Wheldon L. A., Komoriya A., Wayner E. A., Yamada K. M., Humphries M. J. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990 Mar 5;265(7):4020–4024. [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen R., Turunen J. P., Rapola J., Häyry P. Characterization of high endothelial-like properties of peritubular capillary endothelium during acute renal allograft rejection. Am J Pathol. 1990 Sep;137(3):643–651. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Ford W. L. The migration of lymphocytes across specialized vascular endothelium. VI. The migratory behaviour of thoracic duct lymphocytes retransferred from the lymph nodes, spleen, blood, or lymph of a primary recipient. Cell Immunol. 1983 May;78(1):161–173. doi: 10.1016/0008-8749(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Tanaka T., Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. II. Differential regulation of LFA-1 in the interaction between lymphocytes and high endothelial cells. Eur J Immunol. 1991 Mar;21(3):855–858. doi: 10.1002/eji.1830210351. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Washington E. A., Kimpton W. G., Cahill R. N. CD4+ lymphocytes are extracted from blood by peripheral lymph nodes at different rates from other T cell subsets and B cells. Eur J Immunol. 1988 Dec;18(12):2093–2096. doi: 10.1002/eji.1830181235. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van Kemenade P., Weder P., Kuijpers T. W., Figdor C. G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989 Dec 14;342(6251):811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]