Abstract
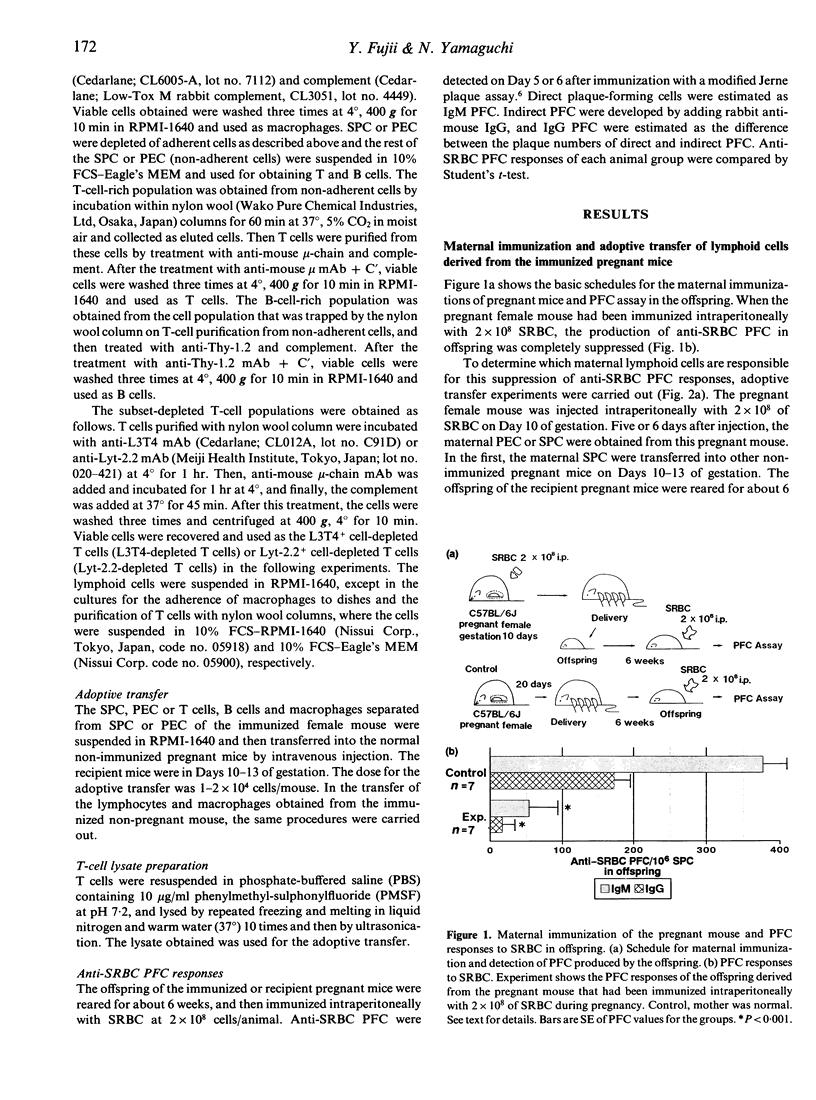
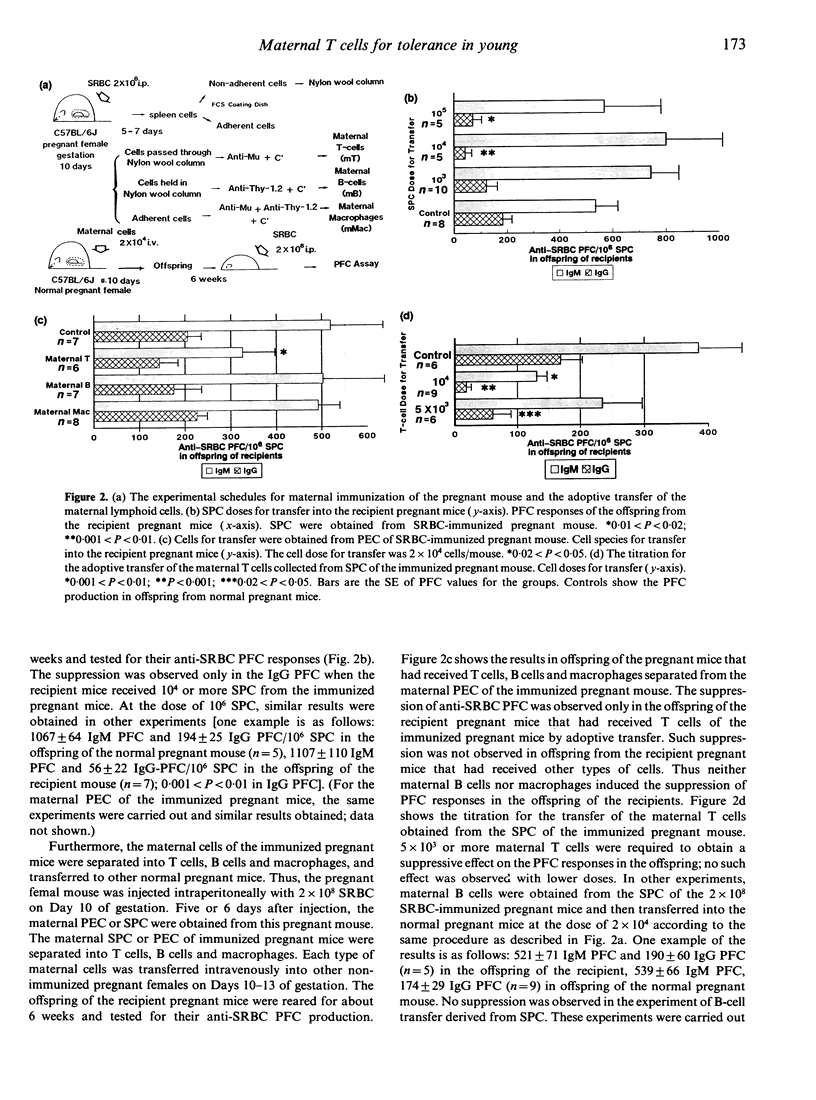
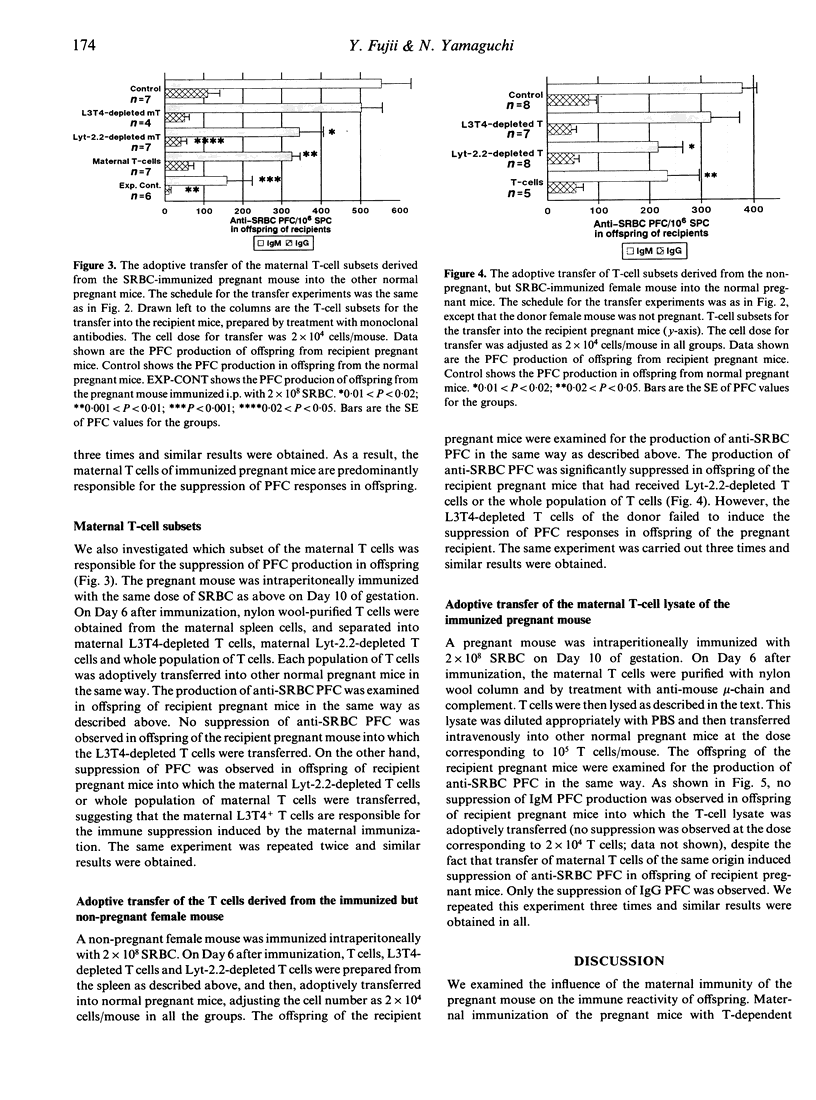
The present study focused on the influence of maternal immunity during pregnancy on the responsiveness of the immune system(s) in offspring. Maternal immunization of pregnant mice with T-dependent foreign antigen sheep red blood cells (SRBC) induced suppression of anti-SRBC plaque-forming cell (PFC) responses in their offspring. We attempted to identify the cell species among the maternal lymphoid cells of the immunized pregnant mice that induced this suppression in their offspring, by separating the maternal cells into T cells, B cells and macrophages, or T-cell subsets, and then adoptively transferring them into other normal pregnant mice. The results demonstrated the following: first, maternal CD4+ T cells of immunized pregnant mice induced immune suppression in their offspring. Second, maternal T cells could be activated during pregnancy in the same fashion as in non-pregnant mice. The T-cell factor(s) for the immune suppression in offspring is produced not only by maternal T cells of immunized pregnant mice but also by T cells activated in non-pregnant mice. Third, cellular organization was required for maternal T cells to induce this immune suppression in their offspring.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Tada T. Generation of T cell repertoire. Two distinct mechanisms for generation of T suppressor cells, T helper cells, and T augmenting cells. J Immunol. 1989 Jan 15;142(2):365–373. [PubMed] [Google Scholar]
- Iwata I., Shimizu S., Yamaguchi N. The effect of maternal antigenic stimulation upon the active immune responsiveness of their offspring: suppression induced by soluble protein antigen, ovalbumin, in mice. Am J Reprod Immunol Microbiol. 1986 Jun;11(2):55–58. doi: 10.1111/j.1600-0897.1986.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Jacoby D. R., Olding L. B., Oldstone M. B. Immunologic regulation of fetal-maternal balance. Adv Immunol. 1984;35:157–208. doi: 10.1016/s0065-2776(08)60576-3. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Shimizu S., Yamaguchi N. Effect of maternal antigenic stimulation on the active immune response of their offspring. Relationship between the immune reactivity of mother mice and the induction of suppression in their young. Scand J Immunol. 1984 Oct;20(4):327–332. doi: 10.1111/j.1365-3083.1984.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Shimizu S., Hara A., Saito T. The effect of maternal antigenic stimulation upon the active immune responsiveness of their offspring. Immunology. 1983 Oct;50(2):229–238. [PMC free article] [PubMed] [Google Scholar]
- Zöller M. Intrathymic T cell repertoire after prenatal trinitrobenzene-sulfonic acid-treatment. Cell Immunol. 1990 Mar;126(1):31–46. doi: 10.1016/0008-8749(90)90298-6. [DOI] [PubMed] [Google Scholar]
- Zöller M. Tolerization during pregnancy: impact on the development of antigen-specific help and suppression. Eur J Immunol. 1988 Dec;18(12):1937–1943. doi: 10.1002/eji.1830181211. [DOI] [PubMed] [Google Scholar]


