Abstract
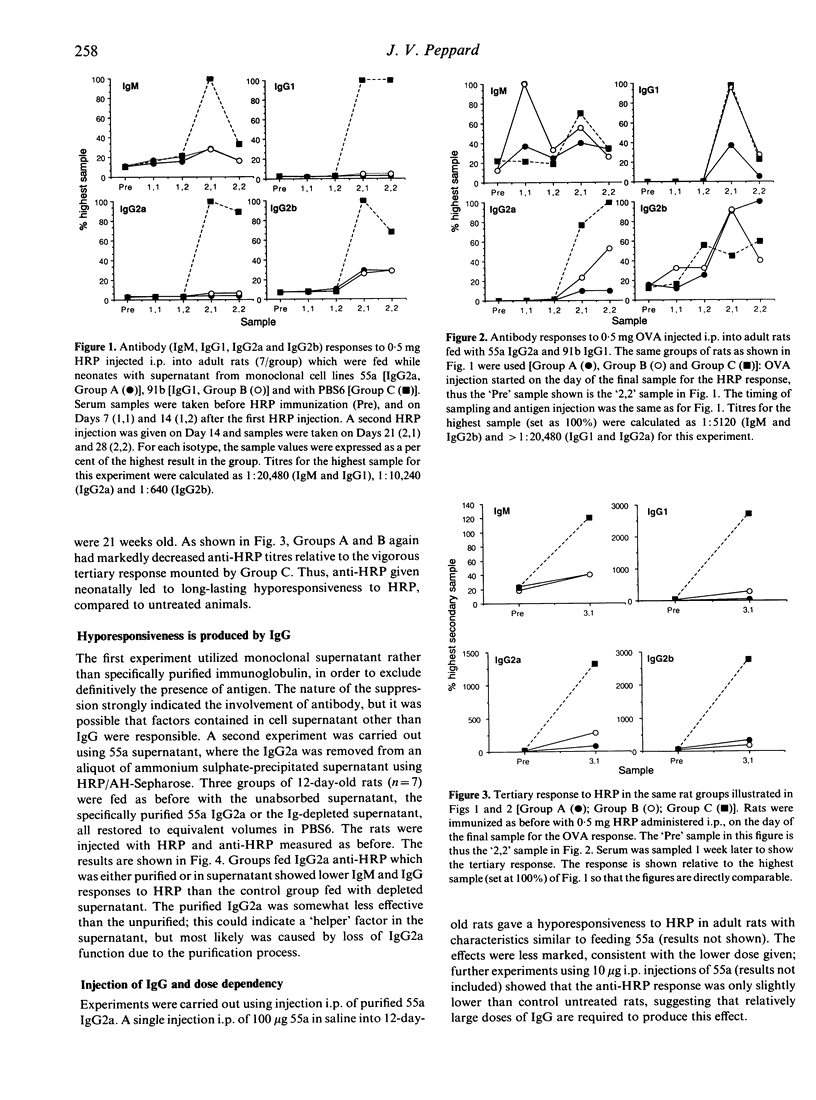
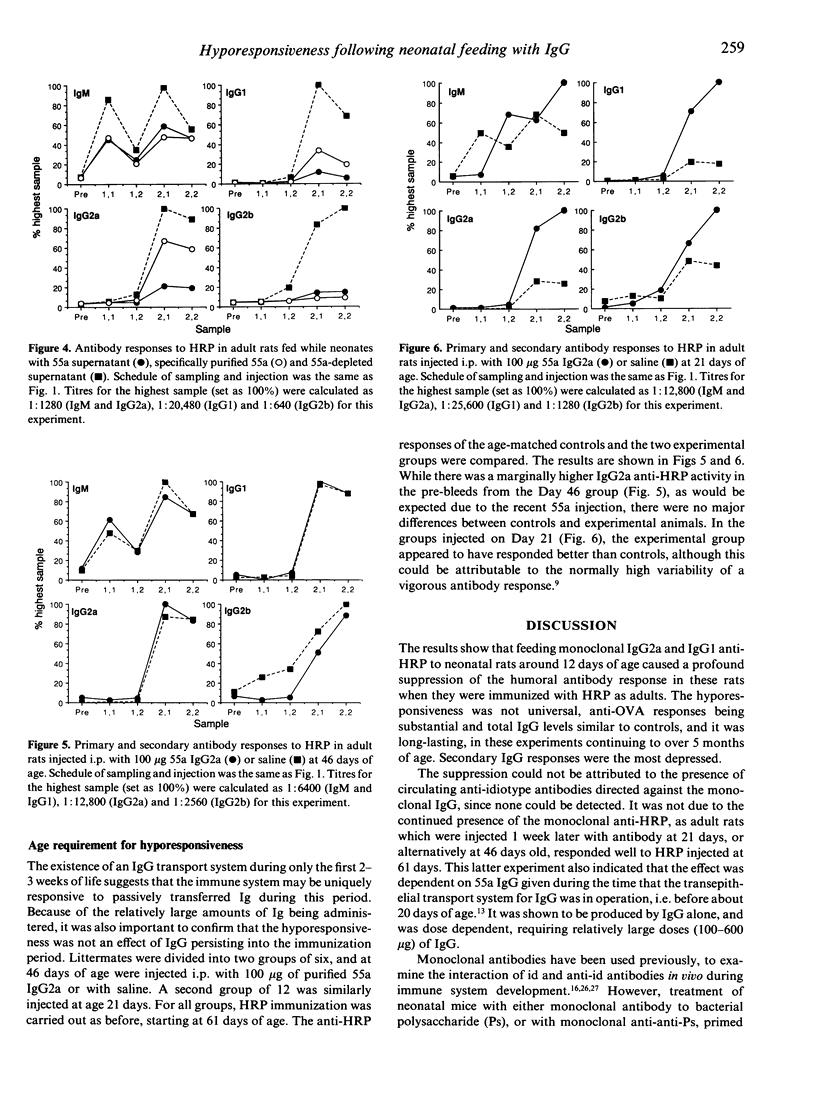
Feeding monoclonal IgG2a or IgG1 anti-horseradish peroxidase (HRP) antibodies to 12-16-day-old neonatal rats caused a profound suppression of the humoral anti-HRP response in these rats as adults. The hyporesponsiveness to HRP was specific and long-lasting (up to 5 months). It was shown to be dose dependent, requiring relatively large doses of IgG (100-600 micrograms) for maximum effect. Secondary IgG (IgG1, IgG2a and IgG2b) responses were most depressed. The effect could be reproduced by i.p. injection of antibody. Hyporesponsiveness was not attributable to circulating antiidiotype antibodies directed against the monoclonal IgG, nor to the continued presence of the monoclonal anti-HRP since rats receiving antibody at or some weeks after the time of weaning and gut 'closure' responded well to subsequent HRP challenge. The effect was thus dependent on IgG administered over the identical period during which the neonatal circulation is rich in maternal IgG supplied via the milk. A direct function for maternal IgG in moulding the immune repertoire of the offspring, as well as providing passive protection, is suggested by these results.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borthistle B. K., Kubo R. T., Brown W. R., Grey H. M. Studies on receptors for IgG on epithelial cells of the rat intestine. J Immunol. 1977 Aug;119(2):471–476. [PubMed] [Google Scholar]
- Davis B. K., Gill T. J., 3rd Decreased antibody response in the offspring of immunized high responder rats. J Immunol. 1975 Oct;115(4):1166–1168. [PubMed] [Google Scholar]
- Dean C. J., Styles J. M., Gyure L. A., Peppard J., Hobbs S. M., Jackson E., Hall J. G. The production of hybridomas from the gut associated lymphoid tissue of tumour bearing rats. I. Mesenteric nodes as a source of IgG producing cells. Clin Exp Immunol. 1984 Aug;57(2):358–364. [PMC free article] [PubMed] [Google Scholar]
- Hall E. P., Gault E. A. Suppression of IgE responses by hyperimmune serum in rats. Immunology. 1986 Dec;59(4):577–581. [PMC free article] [PubMed] [Google Scholar]
- Henry C., Jerne N. K. Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J Exp Med. 1968 Jul 1;128(1):133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs S. M., Jackson L. E., Peppard J. V. Binding of subclasses of rat immunoglobulin G to detergent-isolated Fc receptor from neonatal rat intestine. J Biol Chem. 1987 Jun 15;262(17):8041–8046. [PubMed] [Google Scholar]
- Huxham M., Beck F. Receptor mediated coated vesicle transport of rat IgG across the 11.5 day in vitro rat yolk sac endoderm. Cell Biol Int Rep. 1981 Dec;5(12):1073–1081. doi: 10.1016/s0309-1651(81)80017-3. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. IgE suppression by maternal IgG. Immunology. 1983 Jan;48(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. The development of IgE-suppressive immunocompetence in young animals: influence of exposure to antigen in the presence or absence of maternal immunity. Immunology. 1984 Oct;53(2):365–373. [PMC free article] [PubMed] [Google Scholar]
- Jones E. A., Waldmann T. A. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972 Nov;51(11):2916–2927. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred B., Roelants G. E. Restricted clonal response to DNP in adult offspring of immunized mice: a maternal effect. J Immunol. 1974 Aug;113(2):445–448. [PubMed] [Google Scholar]
- Lalor P. A., Stall A. M., Adams S., Herzenberg L. A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989 Mar;19(3):501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Marcos M. A., Toribio M. L., de la Hera A., Márquez C., Gaspar M. L., Martínez C. Mutual cell interactions and the selection of immune repertoires: implication in autoimmunity. Immunol Today. 1988 Jul-Aug;9(7-8):204–207. doi: 10.1016/0167-5699(88)91214-5. [DOI] [PubMed] [Google Scholar]
- Martinez C., Pereira P., Toribio M. L., Marcos M. A., Bandeira A., de la Hera A., Marquez C., Cazenave P. A., Coutinho A. The participation of B cells and antibodies in the selection and maintenance of T cell repertoires. Immunol Rev. 1988 Jan;101:191–215. doi: 10.1111/j.1600-065x.1988.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Morris B., Morris R., Solari R. The uptake and transmission of protein by neonatal rat enterocytes. J Physiol. 1981 Feb;311:411–420. doi: 10.1113/jphysiol.1981.sp013593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Hall J. G., Robertson D. The transfer of immune complexes from the lumen of the small intestine to the bloodstream in sucking rats. Immunology. 1984 Oct;53(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Mackenzie N. M. Comparison of in vitro binding to enterocyte brush borders of rat IgG subclasses and their transmission in vivo in the rat. Immunology. 1985 Sep;56(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Peri B. A., Rothberg R. M. Specific suppression of antibody production in young rabbit kits after maternal ingestion of bovine serum albumin. J Immunol. 1981 Dec;127(6):2520–2525. [PubMed] [Google Scholar]
- Peri B. A., Rothberg R. M. Transmission of maternal antibody prenatally and from milk into serum of neonatal rabbits. Immunology. 1986 Jan;57(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- Roberts S. A., Turner M. W. Specific suppression of rat IgE responses with milk from immunized females and with feeds of serum antibody. Immunology. 1983 Jan;48(1):195–199. [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol. 1973 Jul;58(1):189–211. doi: 10.1083/jcb.58.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Peppard J. V., Hobbs S. M. Coccidiosis: characterization of antibody responses to infection with Eimeria nieschulzi. Parasite Immunol. 1984 Jan;6(1):1–12. doi: 10.1111/j.1365-3024.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Yeh M., Bona C. A. Idiotype-anti-idiotype network. II. Activation of silent clones by treatment at birth with idiotypes is associated with the expansion of idiotype-specific helper T cells. J Exp Med. 1982 Aug 1;156(2):506–521. doi: 10.1084/jem.156.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J., Yeh M., Bona C. A. Idiotype-anti-idiotype network. II. Activation of silent clones by treatment at birth with idiotypes is associated with the expansion of idiotype-specific helper T cells. J Exp Med. 1982 Aug 1;156(2):506–521. doi: 10.1084/jem.156.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A. Genetic linkage of the cytolytic T lymphocyte repertoire and immunoglobulin heavy chain genes. J Exp Med. 1982 Jul 1;156(1):294–299. doi: 10.1084/jem.156.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Lowy A., HayGlass K., Janeway C. A., Jr, Gurish M., Greene M. I., Benacerraf B. Chronic treatment with rabbit anti-mouse mu-chain antibody alters the characteristic immunoglobulin heavy-chain restriction of murine suppressor T-cell factors. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3846–3850. doi: 10.1073/pnas.81.12.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil M., Sauter H., Paige C., Kearney J. F. In vivo suppression of perinatal multispecific B cells results in a distortion of the adult B cell repertoire. Eur J Immunol. 1986 Sep;16(9):1159–1165. doi: 10.1002/eji.1830160921. [DOI] [PubMed] [Google Scholar]


