Abstract
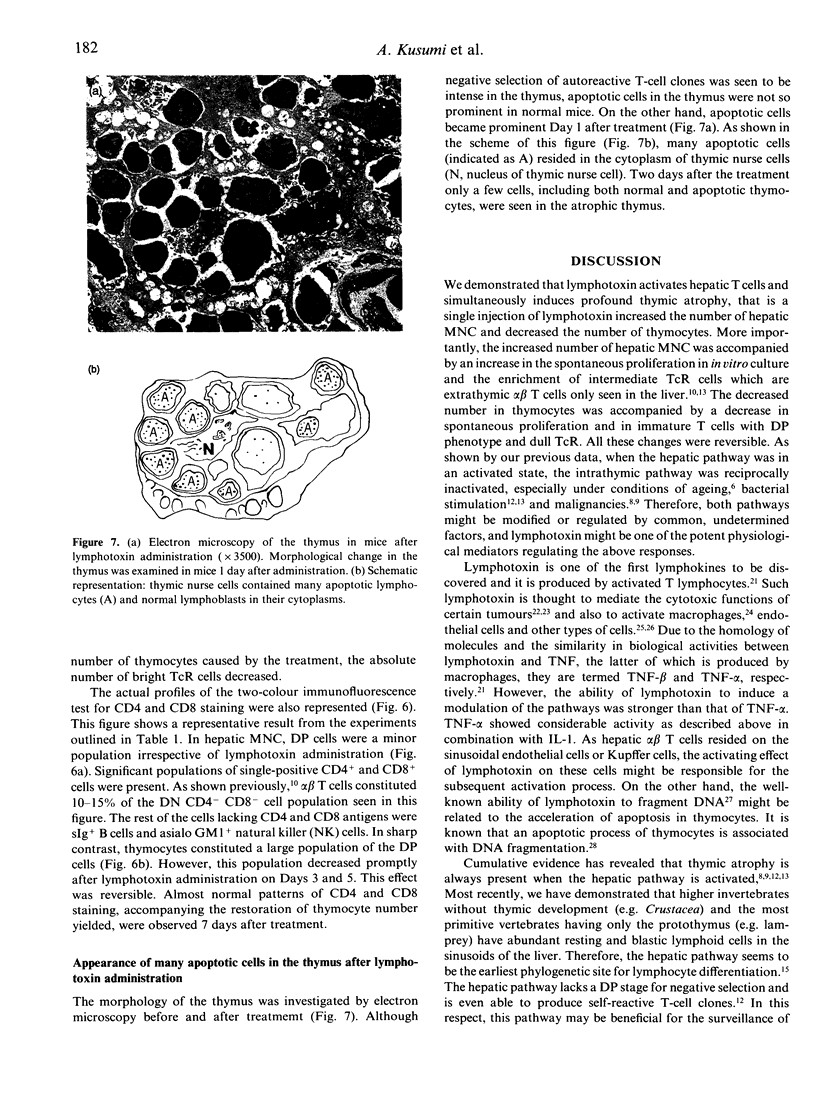
We have recently demonstrated that the liver may be a major site of extrathymic T-cell differentiation. This hepatic pathway was shown to be activated in mice injected with heat-killed bacteria. It is conceivable that the resulting activation of macrophages or lymphocytes, and the production of cytokines may be responsible for a subsequent activation of hepatic T cells. In this context, we investigated the possibility of whether certain cytokines may activate hepatic T cells. It was demonstrated that the administration of lymphotoxin [tumour necrosis factor-beta (TNF-beta)] more than doubled the number of hepatic mononuclear cells (MNC) yielded 3-5 days after the treatment. More strikingly, such treatment induced profound thymic atrophy and resulted in a decrease of more than 95% in the number of thymocytes. Spontaneous proliferation in an in vitro culture of hepatic MNC from treated mice increased, and inversely such activity of thymocytes decreased. The increased number of hepatic MNC was mainly due to an increase in intermediate alpha beta T-cell receptor (TcR) cells, which are extrathymic T cells uniquely seen in the liver. On the other hand, the thymic atrophy was caused by the prompt apoptotic death of dull alpha beta TcR cells with double-positive (DP) CD4+ CD8+ phenotype. These results indicate that lymphotoxin may be one of the factors that activates extrathymic T cells in the liver and at the same time inhibits intrathymic T-cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Kusumi A., Seki S., Ohteki T., Sugiura K., Masuda T., Rikiishi H., Iiai T., Kumagai K. Activation of extrathymic T cells in the liver and reciprocal inactivation of intrathymic T cells by bacterial stimulation. Cell Immunol. 1992 Jun;142(1):125–136. doi: 10.1016/0008-8749(92)90274-s. [DOI] [PubMed] [Google Scholar]
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Ito K., Krecko E. G., Itohara S., Kappes D., Ishida I., Kanagawa O., Janeway C. A., Murphy D. B., Tonegawa S. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Harlan J. M., Adamson J. W. Disparate effects of tumor necrosis factor-alpha/cachectin and tumor necrosis factor-beta/lymphotoxin on hematopoietic growth factor production and neutrophil adhesion molecule expression by cultured human endothelial cells. J Immunol. 1987 Jun 15;138(12):4298–4302. [PubMed] [Google Scholar]
- Fu Y., Paul R. D., Wang Y., Lopez D. M. Thymic involution and thymocyte phenotypic alterations induced by murine mammary adenocarcinomas. J Immunol. 1989 Dec 15;143(12):4300–4307. [PubMed] [Google Scholar]
- Funahashi I., Kawatsu M., Kajikawa T., Takeo K., Asahi T., Kakutani T., Yamashita T., Kawaharada H., Watanabe K. Usefulness of glycosylated recombinant human lymphotoxin for growth inhibition of human and murine solid tumors and experimental metastasis in mice. J Immunother (1991) 1991 Feb;10(1):28–38. doi: 10.1097/00002371-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Utsuyama M., Kurashima C., Hirokawa K. Spontaneous development of organ-specific autoimmune lesions in aged C57BL/6 mice. Clin Exp Immunol. 1989 Oct;78(1):120–126. [PMC free article] [PubMed] [Google Scholar]
- Hemmi H., Nakamura T., Tamura K., Shimizu Y., Kato S., Miki T., Takahashi N., Muramatsu M., Numao N., Sugamura K. Lymphotoxin: induction of terminal differentiation of the human myeloid leukemia cell lines HL-60 and THP-1. J Immunol. 1987 Feb 1;138(3):664–666. [PubMed] [Google Scholar]
- Kishihara K., Yoshikai Y., Matsuzaki G., Mak T. W., Nomoto K. Functional alpha and beta T cell chain receptor messages can be detected in old but not in young athymic mice. Eur J Immunol. 1987 Apr;17(4):477–482. doi: 10.1002/eji.1830170407. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Abo T., Seki S., Kobata T., Yagita H., Okumura K., Kumagai K. Predominant appearance of gamma/delta T lymphocytes in the liver of mice after birth. Eur J Immunol. 1991 Jul;21(7):1733–1740. doi: 10.1002/eji.1830210722. [DOI] [PubMed] [Google Scholar]
- Okuyama R., Abo T., Seki S., Ohteki T., Sugiura K., Kusumi A., Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J Exp Med. 1992 Mar 1;175(3):661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. L., Ruddle N. H. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Powell M. B., Conta B. S., Horowitz M., Ruddle N. H. The differential inhibitory effect of lymphotoxin and immune interferon on normal and malignant lymphoid cells. Lymphokine Res. 1985 Winter;4(1):13–26. [PubMed] [Google Scholar]
- Seki S., Abo T., Masuda T., Ohteki T., Kanno A., Takeda K., Rikiishi H., Nagura H., Kumagai K. Identification of activated T cell receptor gamma delta lymphocytes in the liver of tumor-bearing hosts. J Clin Invest. 1990 Aug;86(2):409–415. doi: 10.1172/JCI114726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Smith M. E., Laudico R., Papermaster B. W. A rapid quantitative assay for lymphotoxin. J Immunol Methods. 1977;14(3-4):243–251. doi: 10.1016/0022-1759(77)90134-x. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Reis M. D., Mak T. W. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986 Dec 4;324(6096):482–485. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]