Abstract
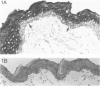
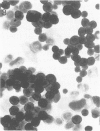
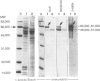
Molecular mimicry between mycobacterial heat-shock protein (hsp) 65 and host tissue antigens have been implicated in the autoimmune pathogenesis of certain idiopathic diseases. Here, we demonstrated that two of our previously characterized monoclonal antibodies (mAb), Ne5 and Nd4 that were directed to a carboxy-terminal epitope on the mycobacterial hsp 65, specifically cross-reacted with suprabasal cytokeratin of the normal human skin. These mAb also showed similar keratin staining of hair follicle epithelia and produced no reaction with other dermal components. Both mAb strongly stained the cytoplasm of the majority of freshly isolated epidermal keratinocytes from the normal human skin. None of these mAb showed staining with human HeLa cells and with human skin fibroblasts. Immunoblotting using total keratin extract prepared from isolated epidermal keratinocytes revealed that mAb Ne5 and Nd4 specifically reacted with a molecular size of 65,000-67,000 MW keratin protein(s) and such reactivity was not observed from cytoskeletal proteins extracted from HeLa cells and skin fibroblasts. Comparison of immunoblotting reactivity with conventional anti-cytokeratin mAb further revealed that mAb Ne5/Nd4 recognized a 65,000-67,000 MW molecular-sized protein corresponding to cytokeratin 1/2 from the same keratinocyte extract as anti-cytokeratin mAb. Preincubation of mAb Ne5/Nd4 with the purified mycobacterial hsp 65 abolished this keratin cross-reactivity in both immunohistochemistry and immunoblotting. Moreover, these mAb showed no keratin staining in lesional psoriatic skin and also reacted weakly with cultured epidermal keratinocytes. Since mAb Ne5/Nd4 specifically recognized a 67,000-65,000 MW molecular-sized protein(s) derived from epidermal keratinocytes and the known characteristics of epidermal cytokeratin 1/2 appeared to be consistent with present results, we concluded that Ne5/Nd4 cross-reactive protein(s) in the human epidermis is suprabasal cytokeratin 1/2. Comparison of the previously mapped Ne5/Nd4 epitope region of amino acid residues 525-540 of the mycobacterial hsp 65 with the entire sequence of human 65,000 MW keratin revealed that a stretch of nine amino acids of the Ne5/Nd4 epitope sequence resembled certain regions of the carboxy-terminus of the human 65,000 MW keratin. This similarity of the mycobacterial hsp 65 probably contributes to the cytokeratin cross-reactive epitope. Our results presented here demonstrate direct evidence of immunological cross-reactivity between mycobacterial hsp 65 and human epidermal cytokeratin 1/2. We speculate that Ne5/Nd4 cross-reactive epitope of epidermal cytokeratins might be an important target for skin diseases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguas A., Esaguy N., Sunkel C. E., Silva M. T. Cross-reactivity and sequence homology between the 65-kilodalton mycobacterial heat shock protein and human lactoferrin, transferrin, and DR beta subsets of major histocompatibility complex class II molecules. Infect Immun. 1990 May;58(5):1461–1470. doi: 10.1128/iai.58.5.1461-1470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Aoki S., Yaoita H., Kitajima Y. An elevated level of autoantibodies against 48- to 50-kd keratins in the serum of patients with psoriasis. J Invest Dermatol. 1989 Feb;92(2):179–183. doi: 10.1111/1523-1747.ep12276700. [DOI] [PubMed] [Google Scholar]
- Bos J. D. The pathomechanisms of psoriasis; the skin immune system and cyclosporin. Br J Dermatol. 1988 Feb;118(2):141–155. doi: 10.1111/j.1365-2133.1988.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Das P. K., Rambukkana A., Baas J. G., Groothuis D. G., Halperin M. Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Feb;28(2):379–382. doi: 10.1128/jcm.28.2.379-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Bonitz P., Sun T. T. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984 Apr;98(4):1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Jenner P. J., Colston M. J., Bacon P. A. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990 Mar 1;171(3):831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge E. M., Sylvestre Y. R., Freedberg I. M., Blumenberg M. Evolution of keratin genes: different protein domains evolve by different pathways. J Mol Evol. 1987;24(4):319–329. doi: 10.1007/BF02134130. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Naafs B., Kolk A. H., Chin A Lien R. A., Faber W. R., Van Dijk G., Kuijper S., Stolz E., Van Joost T. Anti-Mycobacterium leprae monoclonal antibodies cross-react with human skin: an alternative explanation for the immune responses in leprosy. J Invest Dermatol. 1990 May;94(5):685–688. doi: 10.1111/1523-1747.ep12876264. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Moesker O., Kant A., Huysmans A., Haag D., Jap P. H., Herman C. J., Vooijs G. P. Antibodies to intermediate filament proteins in the immunohistochemical identification of human tumours: an overview. Histochem J. 1983 Jul;15(7):691–713. doi: 10.1007/BF01002988. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Chand A., Baas J. G., Groothuis D. G., Kolk A. H. Subcellular distribution of monoclonal antibody defined epitopes on immunodominant Mycobacterium tuberculosis proteins in the 30-kDa region: identification and localization of 29/33-kDa doublet proteins on mycobacterial cell wall. Scand J Immunol. 1991 Jun;33(6):763–775. doi: 10.1111/j.1365-3083.1991.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Faber W. R. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions. Evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992 Jul;36(1):35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Yong S., Das P. K. Identification of a novel B-cell epitope of restricted specificity on the hsp 65-kDa protein of Mycobacterium tuberculosis. FEMS Microbiol Immunol. 1991 Feb;3(1):39–45. doi: 10.1111/j.1574-6968.1991.tb04161.x. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A., Idler W. W., Johnson L. D., Steven A. C., Roop D. R. Amino acid sequences of mouse and human epidermal type II keratins of Mr 67,000 provide a systematic basis for the structural and functional diversity of the end domains of keratin intermediate filament subunits. J Biol Chem. 1985 Jun 10;260(11):7142–7149. [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Vaughan J. H., Kouri T., Petersen J., Roudier J., Rhodes G. H. On the etiology of rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;74:19–28. doi: 10.3109/03009748809102935. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- de Graeff-Meeder E. R., Voorhorst M., van Eden W., Schuurman H. J., Huber J., Barkley D., Maini R. N., Kuis W., Rijkers G. T., Zegers B. J. Antibodies to the mycobacterial 65-kd heat-shock protein are reactive with synovial tissue of adjuvant arthritic rats and patients with rheumatoid arthritis and osteoarthritis. Am J Pathol. 1990 Nov;137(5):1013–1017. [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Cohen I. Antigenic mimicry between mycobacteria and cartilage proteoglycans: the model of adjuvant arthritis. Concepts Immunopathol. 1987;4:144–170. [PubMed] [Google Scholar]