Abstract
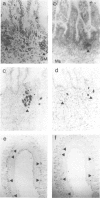
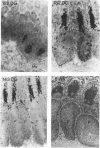
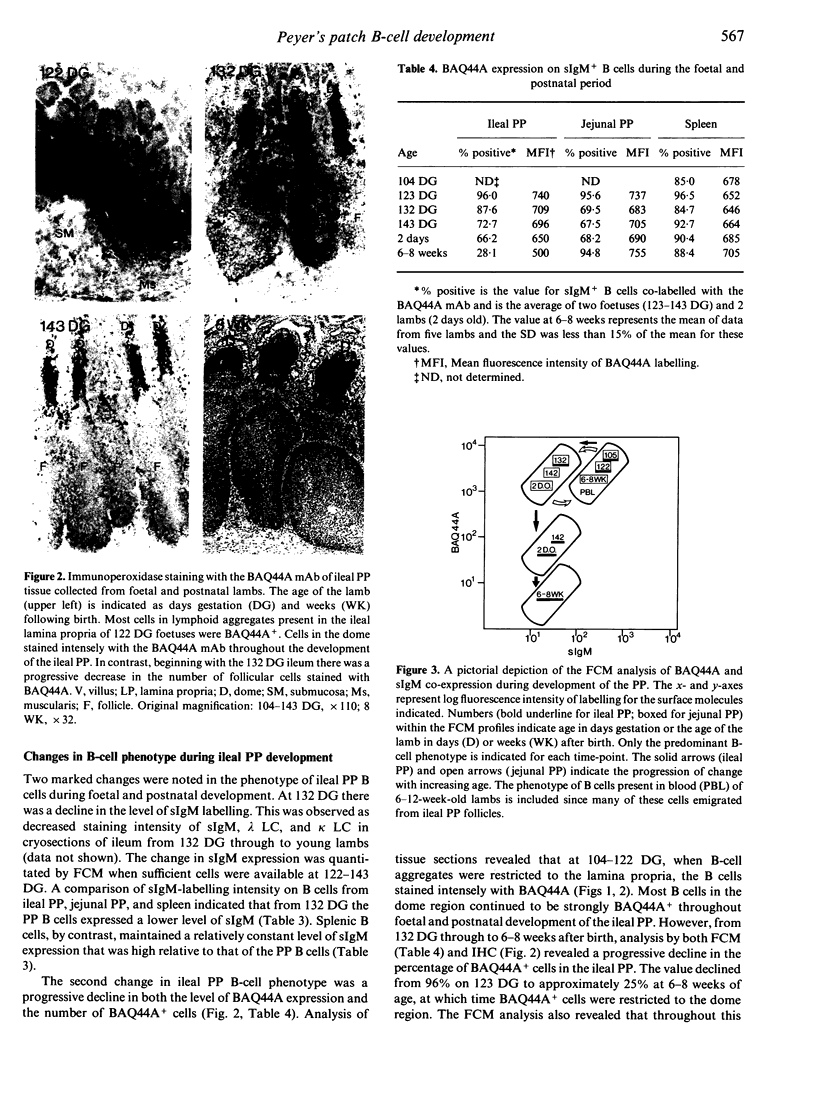
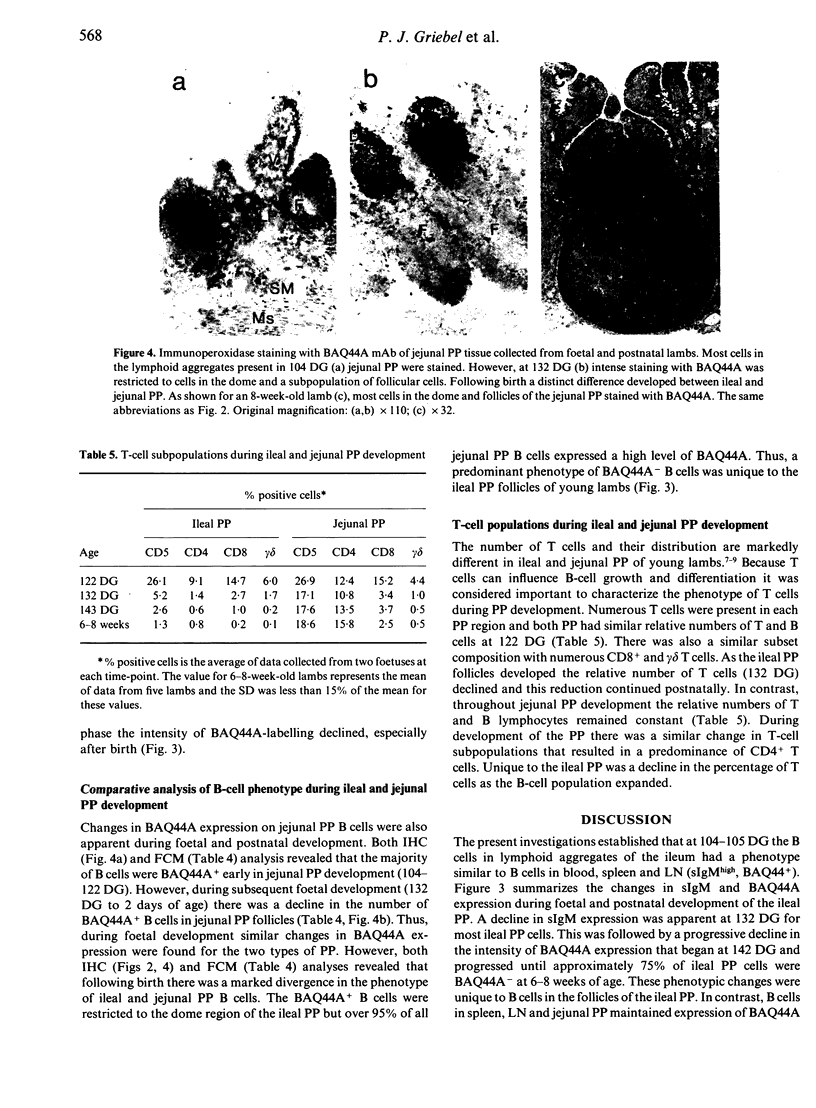
Changes in B-cell phenotype during development of ileal and jejunal Peyer's patches (PP) of sheep were investigated using flow cytometry and immunoperoxidase-stained cryosections. On Day 104 of gestation (term at 150 days) B-cell clusters were identified in the lamina propria of the ileum. These clusters were composed of cells that expressed surface IgM (sIgM), lambda or kappa light chain, and BAQ44A, a B-cell differentiation molecule. No cells in the clusters stained for terminal deoxynucleotide transferase. On Day 132 gestation, a change was evident in the phenotype of ileal PP B cells. Most B cells expressed a reduced level of sIgM and 20% were BAQ44A-. The B cells in the dome region were BAQ44A+ but few BAQ44A+ cells were present in the follicles. At 6-8 weeks of age BAQ44A+ cells were restricted to the dome region of the ileal PP; flow cytometric analysis confirmed that 25% of B cells isolated from the dome/follicle complex were BAQ44A+. Thus, the primordial PP was populated with B cells that were phenotypically similar to circulating B cells (sIgMhigh, BAQ44A+). After 132 days gestation, the predominant B-cell phenotype in the ileal PP changed to sIgMlow and BAQ44A-. This phenotypic change could be the result of either early immigrant B-cell differentiation or subsequent colonization by sIgMlow BAQ44A- B cells. The phenotypic changes of ileal PP follicular B cells were not complete until after birth and different phenotypic changes were observed in follicles of the jejunal PP of young lambs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandersen M., Hein W. R., Landsverk T., McClure S. Distribution of lymphocyte subsets in the large intestinal lymphoid follicles of lambs. Immunology. 1990 Jul;70(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Rouse R. V., Coffman R. L., Nottenburg C. N., Hardy R. R., Weissman I. L. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982 Dec;129(6):2698–2707. [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Griebel P. J., Davis W. C., Reynolds J. D. An analysis of the growth and differentiation of B cells isolated from follicles of the ileal Peyer's patch of sheep. Immunology. 1992 Apr;75(4):601–607. [PMC free article] [PubMed] [Google Scholar]
- Griebel P. J., Davis W. C., Reynolds J. D. Negative signaling by surface IgM on B cells isolated from ileal Peyer's patch follicles of sheep. Eur J Immunol. 1991 Sep;21(9):2281–2284. doi: 10.1002/eji.1830210943. [DOI] [PubMed] [Google Scholar]
- Halleraker M., Landsverk T., Nicander L. Organization of ruminant Peyer's patches as seen with enzyme histochemical markers of stromal and accessory cells. Vet Immunol Immunopathol. 1990 Sep;26(1):93–104. doi: 10.1016/0165-2427(90)90135-f. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Dudler L., Mackay C. R. Surface expression of differentiation antigens on lymphocytes in the ileal and jejunal Peyer's patches of lambs. Immunology. 1989 Nov;68(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Hardy R. R., Gallatin W. M., Weissman I. L., Butcher E. C. Antigen-induced changes in B cell subsets in lymph nodes: analysis by dual fluorescence flow cytofluorometry. Eur J Immunol. 1986 Jul;16(7):829–834. doi: 10.1002/eji.1830160718. [DOI] [PubMed] [Google Scholar]
- Larsen H. J., Landsverk T. Distribution of T and B lymphocytes in jejunal and ileocaecal Peyer's patches of lambs. Res Vet Sci. 1986 Jan;40(1):105–111. [PubMed] [Google Scholar]
- Larsen R. A., Monaghan M. L., Park Y. H., Hamilton M. J., Ellis J. A., Davis W. C. Identification and characterization of monoclonal antibodies reactive with bovine, caprine and ovine T-lymphocyte determinants by flow microfluorimetry. Vet Immunol Immunopathol. 1990 Jun;25(2):195–208. doi: 10.1016/0165-2427(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Hein W. R., Brown M. H., Matzinger P. Unusual expression of CD2 in sheep: implications for T cell interactions. Eur J Immunol. 1988 Nov;18(11):1681–1688. doi: 10.1002/eji.1830181105. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., Dudler L., Bordmann G., Leiserson W. M., Gerber H. A., Reynolds J., Trnka Z. Differentiation of B lymphocytes in sheep. I. Phenotypic analysis of ileal Peyer's patch cells and the demonstration of a precursor population for sIg+ cells in the ileal Peyer's patches. Immunology. 1984 Nov;53(3):515–523. [PMC free article] [PubMed] [Google Scholar]
- Motyka B., Reynolds J. D. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer's patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur J Immunol. 1991 Aug;21(8):1951–1958. doi: 10.1002/eji.1830210825. [DOI] [PubMed] [Google Scholar]
- Pabst R., Geist M., Rothkötter H. J., Fritz F. J. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988 Jul;64(3):539–544. [PMC free article] [PubMed] [Google Scholar]
- Parsons K. R., Howard C. J., Jones B. V., Sopp P. Investigation of bovine gut associated lymphoid tissue (GALT) using monoclonal antibodies against bovine lymphocytes. Vet Pathol. 1989 Sep;26(5):396–408. doi: 10.1177/030098588902600505. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D. Evidence of extensive lymphocyte death in sheep Peyer's patches. I. A comparison of lymphocyte production and export. J Immunol. 1986 Mar 15;136(6):2005–2010. [PubMed] [Google Scholar]
- Reynolds J. D., Kennedy L., Peppard J., Pabst R. Ileal Peyer's patch emigrants are predominantly B cells and travel to all lymphoid tissues in sheep. Eur J Immunol. 1991 Feb;21(2):283–289. doi: 10.1002/eji.1830210207. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D. Mitotic rate maturation in the Peyer's patches of fetal sheep and in the bursa of Fabricius of the chick embryo. Eur J Immunol. 1987 Apr;17(4):503–507. doi: 10.1002/eji.1830170411. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The effect of antigen on the development of Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):1–6. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The evolution and involution of Peyer's patches in fetal and postnatal sheep. Eur J Immunol. 1983 Aug;13(8):627–635. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Yutoku M., Grossberg A., Pressman D., Herzenberg L. A. A surface membrane determinant shared by subpopulations of thymocytes and B lymphocytes. J Immunol. 1975 Aug;115(2):508–512. [PubMed] [Google Scholar]
- Yutoku M., Grossberg A. L., Pressman D. A cell surface antigenic determinant present on mouse plasmacytes and only about half of mouse thymocytes. J Immunol. 1974 May;112(5):1774–1781. [PubMed] [Google Scholar]