Abstract
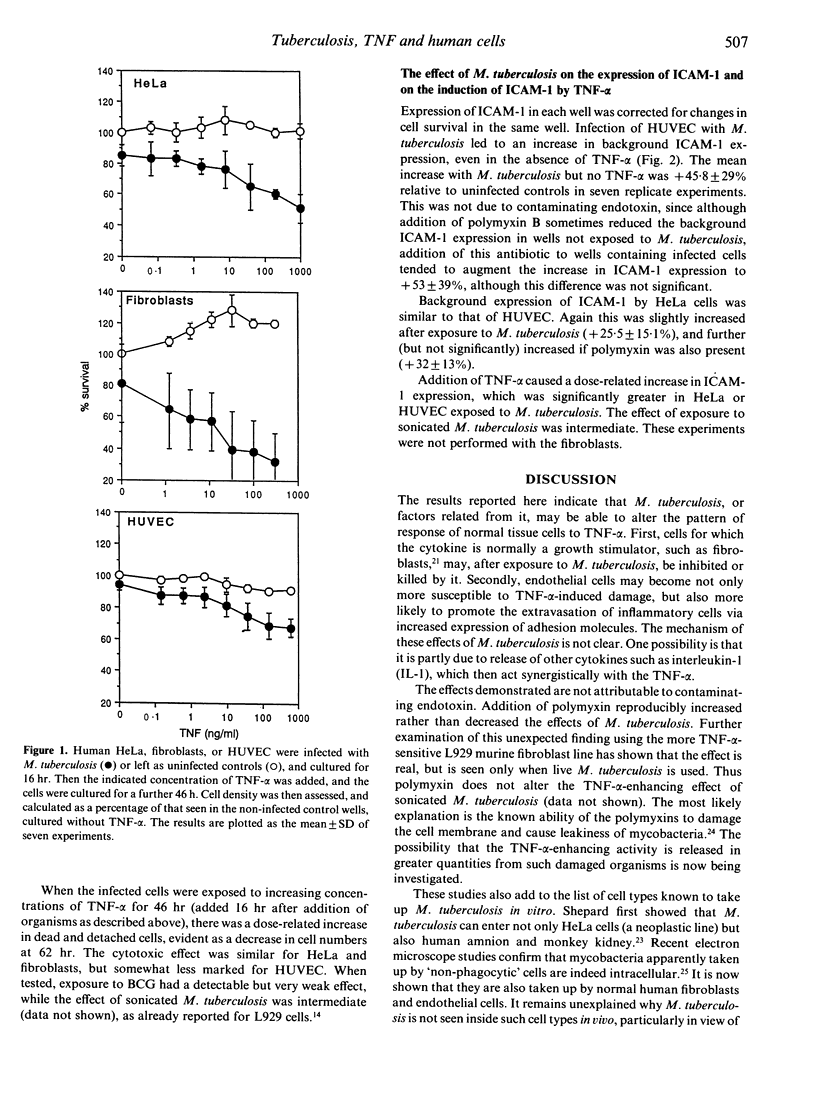
It has previously been shown that the inherently tumour necrosis factor-alpha (TNF-alpha)-sensitive L929 murine fibroblast cell line becomes much more sensitive to the cytotoxic effect of this cytokine after exposure to Mycobacterium tuberculosis in culture. In this study it is now shown that normal human cells of types likely to be involved in tuberculous lesions are affected in a similar way. Growth of normal human fibroblasts is usually stimulated by TNF-alpha in vitro, but after exposure to M. tuberculosis or to extracts of this organism, these cells are killed rather than stimulated by subsequent exposure to TNF-alpha. Similarly, human endothelial cells become susceptible to doses to TNF-alpha which do not normally affect viability. Moreover this enhancement of sensitivity to TNF-alpha is not confined to its toxicity. Endothelial cells and HeLa cells exposed to M. tuberculosis express increased levels of ICAM-1 after subsequent exposure to TNF-alpha, implying synergy between the two stimuli. It is suggested that these effects contribute to the ability of M. tuberculosis to distort the normal protective role of TNF-alpha so that the cytokine becomes detrimental to the host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austgulen R., Espevik T., Nissen-Meyer J. Fibroblast growth-stimulatory activity released from human monocytes. The contribution of tumour necrosis factor. Scand J Immunol. 1987 Dec;26(6):621–629. doi: 10.1111/j.1365-3083.1987.tb02297.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Boddingius J. The occurrence of Mycobacterium leprae within axons of peripheral nerves. Acta Neuropathol. 1974 Mar 26;27(3):257–270. doi: 10.1007/BF00687635. [DOI] [PubMed] [Google Scholar]
- Bull H. A., Pittilo R. M., Woolf N., Machin S. J. The effect of nicotine on human endothelial cell release of prostaglandins and ultrastructure. Br J Exp Pathol. 1988 Jun;69(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Denis M. Killing of Mycobacterium tuberculosis within human monocytes: activation by cytokines and calcitriol. Clin Exp Immunol. 1991 May;84(2):200–206. doi: 10.1111/j.1365-2249.1991.tb08149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri M. M., Weddell A. G., Rees R. J. Infection of murine striated muscle with Mycobacterium leprae: a study by light and electron microscopy. J Pathol. 1972 Feb;106(2):73–80. doi: 10.1002/path.1711060203. [DOI] [PubMed] [Google Scholar]
- Filley E. A., Rook G. A. Effect of mycobacteria on sensitivity to the cytotoxic effects of tumor necrosis factor. Infect Immun. 1991 Aug;59(8):2567–2572. doi: 10.1128/iai.59.8.2567-2572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley N., Lambert C., McNicol M., Johnson N., Rook G. A. An inhibitor of the toxicity of tumour necrosis factor in the serum of patients with sarcoidosis, tuberculosis and Crohn's disease. Clin Exp Immunol. 1990 Jun;80(3):395–399. doi: 10.1111/j.1365-2249.1990.tb03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach H., Lieberman H., Bach R., Godman G., Brett J., Stern D. Enhanced responsiveness of endothelium in the growing/motile state to tumor necrosis factor/cachectin. J Exp Med. 1989 Sep 1;170(3):913–931. doi: 10.1084/jem.170.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Fiers W., North R. J. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988 Mar 1;167(3):1067–1085. doi: 10.1084/jem.167.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Kirby J. A., Shenton B. K., Givan A. L., Lennard T. W. Human endothelial cells: effect of TNF-alpha on peripheral blood mononuclear cell adhesion. Immunology. 1991 May;73(1):71–76. [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Henrotte J. G., David H. L. Colistin (polymyxin E)--induced cell leakage in Mycobacterium aurum. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Mar;263(4):548–551. doi: 10.1016/s0176-6724(87)80198-0. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Foley N. M., Meager A. What mediates the immunopathological component of the immune response to Mycobacterium tuberculosis? Can it be switched off? Bull Int Union Tuberc Lung Dis. 1990 Jun-Sep;65(2-3):23–26. [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J., Pottinger B. E., Woo P., Black C. M., Loizou S., Byron M. A., Pearson J. D. Measurement and characterisation of circulating anti-endothelial cell IgG in connective tissue diseases. Clin Exp Immunol. 1988 Jun;72(3):450–456. [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. A comparison of the growth of selected mycobacteria in HeLa, monkey kidney, and human amnion cells in tissue culture. J Exp Med. 1958 Feb 1;107(2):237–246. doi: 10.1084/jem.107.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Faccioli L. H. Tumor necrosis factor (cachectin) mediates induction of cachexia by cord factor from mycobacteria. Infect Immun. 1988 Dec;56(12):3067–3071. doi: 10.1128/iai.56.12.3067-3071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Attiyah R., Moreno C., Rook G. A. TNF alpha-mediated tissue damage in mouse footpads primed with mycobacterial preparations. Res Immunol. 1992 Jul-Aug;143(6):601–610. doi: 10.1016/0923-2494(92)80041-i. [DOI] [PubMed] [Google Scholar]
- al Attiyah R., Rosen H., Rook G. A. A model for the investigation of factors influencing haemorrhagic necrosis mediated by tumour necrosis factor in tissue sites primed with mycobacterial antigen preparations. Clin Exp Immunol. 1992 Jun;88(3):537–542. doi: 10.1111/j.1365-2249.1992.tb06483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


