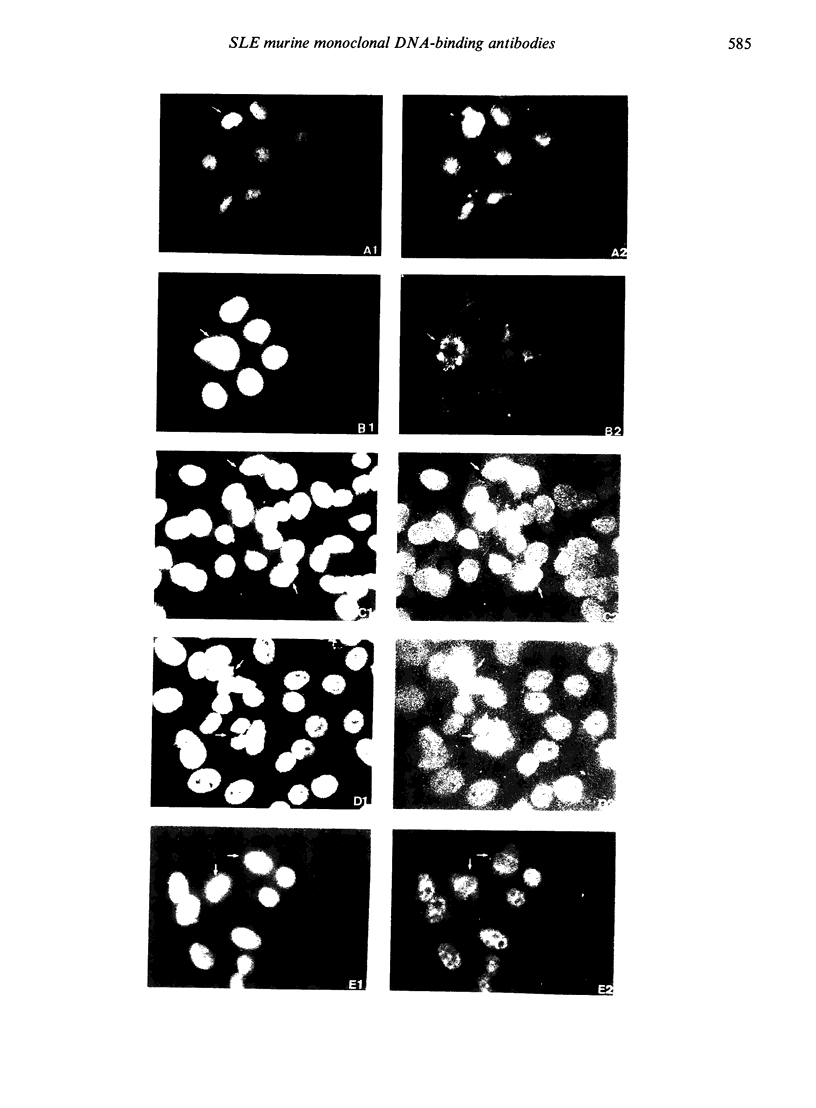
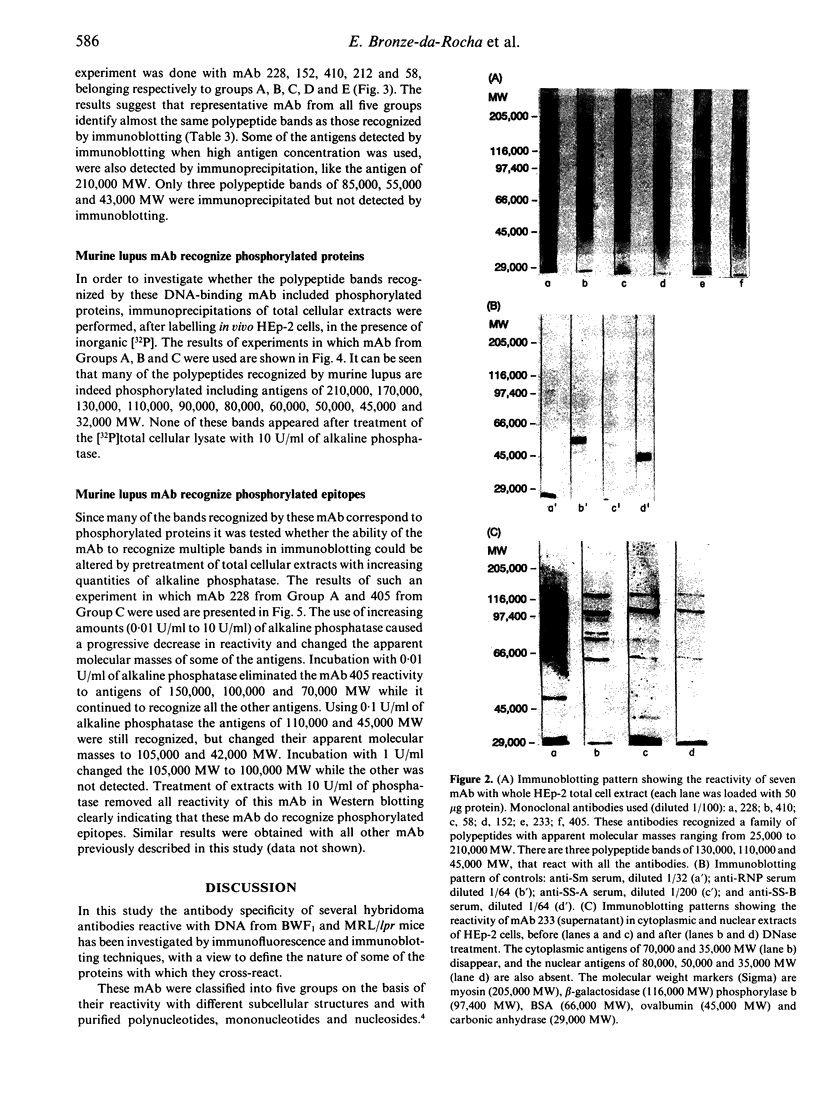
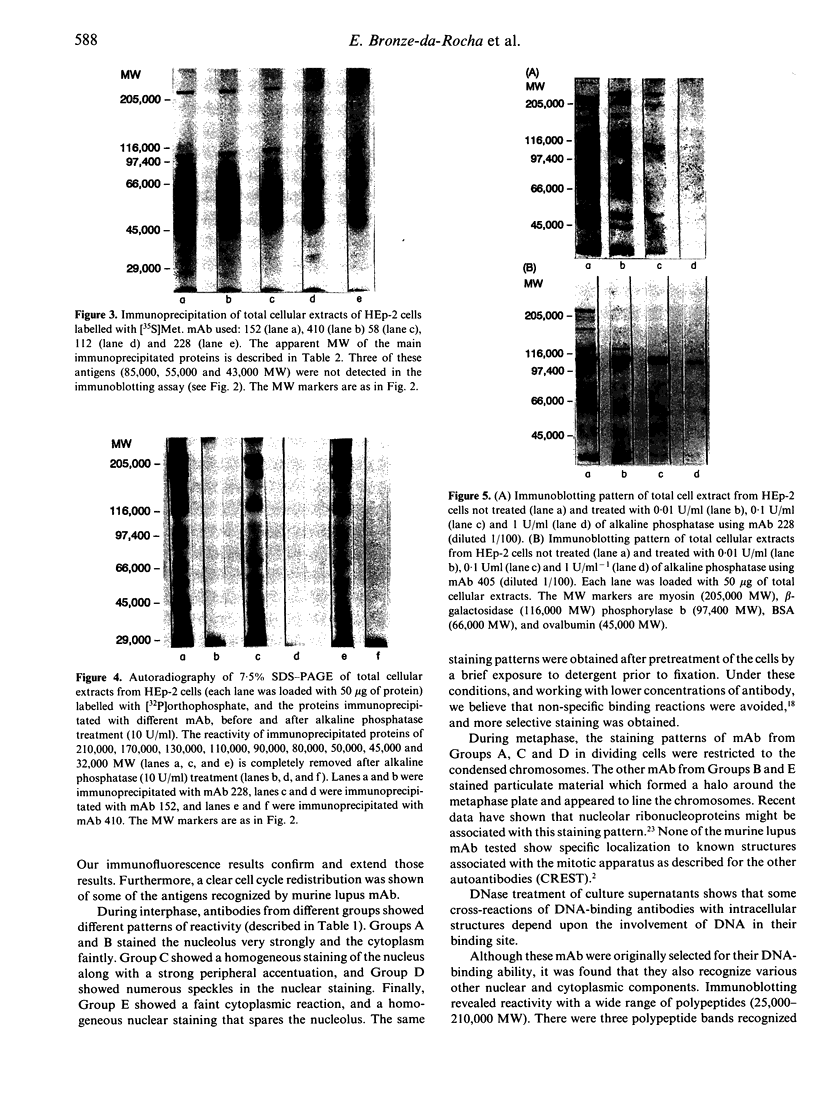
Abstract
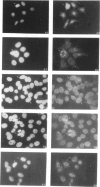
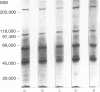
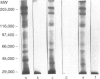
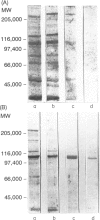
The immunological basis for the production of autoantibodies characteristic of systemic lupus erythematosus (SLE) against a wide range of antigens remains obscure. The specificity of (NZB x NZW)F1 (BWF1) or MRL/Mp-lpr/lpr (MRL/lpr) mouse monoclonal antibodies (mAb) was examined by immunofluorescence, immunoblotting and immunoprecipitation techniques. Using non-synchronized HEp-2 cells as substrate, the murine mAb were classified by indirect immunofluorescence into five groups on the basis of their staining patterns of subcellular components in interphase and mitotic stages of the cell cycle. The nature of the antigens recognized by the murine lupus was assessed by immunoblotting experiments in total, cytoplasmic and nuclear cell extracts from HEp-2 cells. The six antibodies used recognized in total cell extracts a range of polypeptides with apparent molecular weights from 25,000 to 210,000. Three polypeptides of 130,000, 110,000 and 45,000 MW were recognized by all six antibodies in both nuclear and cytoplasmic extracts. Immunoprecipitation of total cellular extracts labelled with [35S]methionine showed almost the same pattern as obtained in the immunoblotting assay. The labelling in vivo of HEp-2 cells with [32P], followed by the immunoprecipitation of the [32P]cell lysate showed that these mAb recognized phosphorylated proteins. The progressive decrease in reactivity of these mAb following treatment with higher concentrations of alkaline phosphatase in both [32P]cell lysate or nitrocellulose membranes indicates that these mAb recognize phosphorylated epitopes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlakha R. C., Sahasrabuddhe C. G., Wright D. A., Lindsey W. F., Rao P. N. Localization of mitotic factors on metaphase chromosomes. J Cell Sci. 1982 Apr;54:193–206. doi: 10.1242/jcs.54.1.193. [DOI] [PubMed] [Google Scholar]
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini P., Chu J. L., Okolo C., Elkon K. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990 Feb;85(2):325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman K., Termaat R. M., de Jong J., van den Brink H. G., Berden J. H., Smeenk R. J. Cross-reactive binding patterns of monoclonal antibodies to DNA are often caused by DNA/anti-DNA immune complexes. Res Immunol. 1989 Jun-Aug;140(5-6):595–612. doi: 10.1016/0923-2494(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Epitopic targets for autoantibodies in systemic lupus erythematosus and Sjögren's syndrome. Curr Opin Rheumatol. 1989 Oct;1(3):376–381. doi: 10.1097/00002281-198901030-00022. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier T., Dauphin-Villemant C., André C., Masson C., Arnoult J., Hernandez-Verdun D. Identification and characterization of a new set of nucleolar ribonucleoproteins which line the chromosomes during mitosis. Exp Cell Res. 1992 May;200(1):5–15. doi: 10.1016/s0014-4827(05)80065-5. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan A., Buchanan R. R., Lew A. M., Olsen I., Staines N. A. Five groups of antigenic determinants on DNA identified by monoclonal antibodies from (NZB X NZW)F1 and MRL/Mp-lpr/lpr mice. Immunology. 1985 May;55(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Isenberg D. A., Naparstek Y., Rauch J., Duggan D., Khiroya R., Staines N. A., Schattner A. Shared idiotypes are expressed on mouse and human anti-DNA autoantibodies. Immunology. 1985 Nov;56(3):393–399. [PMC free article] [PubMed] [Google Scholar]
- Netter H. J., Guldner H. H., Szostecki C., Will H. Major autoantigenic sites of the (U1) small nuclear ribonucleoprotein-specific 68-kDa protein. Scand J Immunol. 1990 Aug;32(2):163–176. doi: 10.1111/j.1365-3083.1990.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Grudier J. P., Gilkeson G. S. A role for immunogenic DNA in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1990 Feb;33(2):153–159. doi: 10.1002/art.1780330202. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Zhao J. Y., Ganju R. K., Ashorn C. L. Monoclonal antibody against the centrosome. J Cell Sci. 1989 May;93(Pt 1):63–69. doi: 10.1242/jcs.93.1.63. [DOI] [PubMed] [Google Scholar]
- Ravirajan C. T., Staines N. A. Involvement in lupus disease of idiotypes Id.F-423 and Id.IV-228 defined, respectively, upon foetal and adult MRL/Mp-lpr/lpr DNA-binding monoclonal autoantibodies. Immunology. 1991 Oct;74(2):342–347. [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Chan E. K., Sullivan K. F., Rubin R. L. Antinuclear antibodies (ANAs): diagnostically specific immune markers and clues toward the understanding of systemic autoimmunity. Clin Immunol Immunopathol. 1988 May;47(2):121–141. doi: 10.1016/0090-1229(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandré D. D., Borisy G. G. Anaphase onset and dephosphorylation of mitotic phosphoproteins occur concomitantly. J Cell Sci. 1989 Oct;94(Pt 2):245–258. doi: 10.1242/jcs.94.2.245. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Watts R. A., Ravirajan C. T., Staines N. A., Isenberg D. A. A human fetal monoclonal DNA-binding antibody shares idiotypes with fetal and adult murine monoclonal DNA-binding antibodies. Immunology. 1990 Mar;69(3):348–354. [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]