Abstract
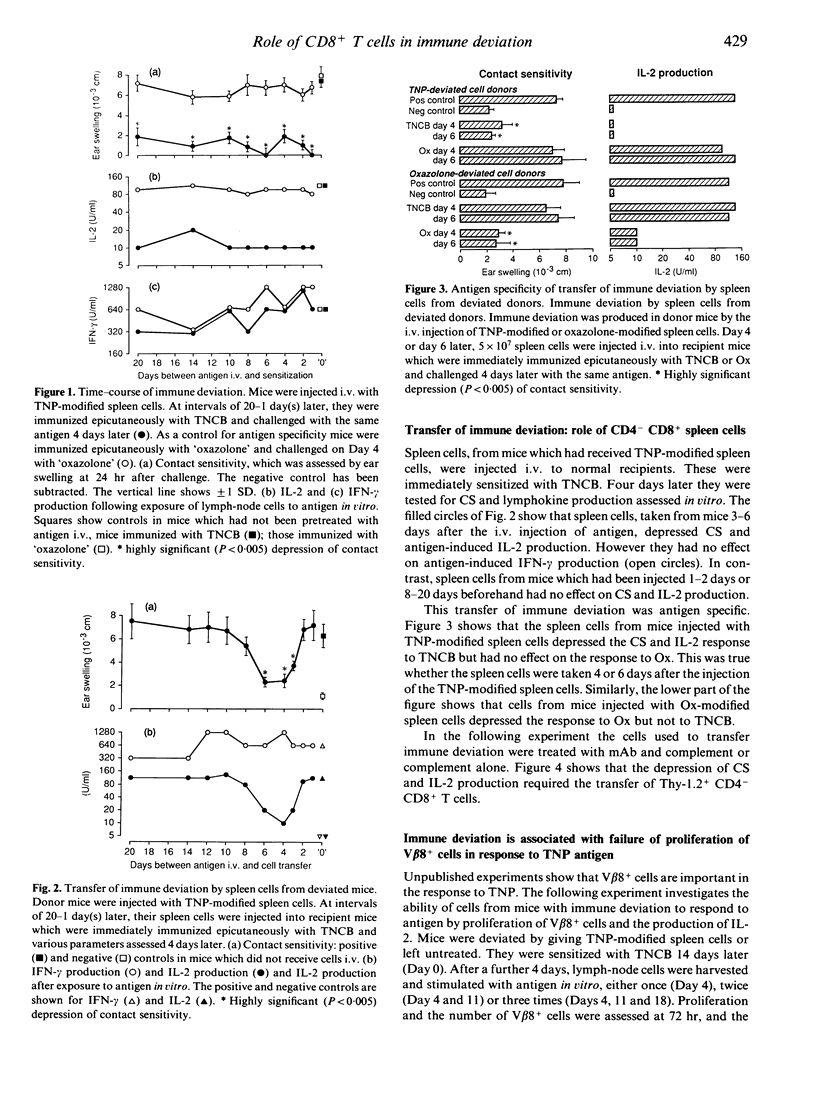
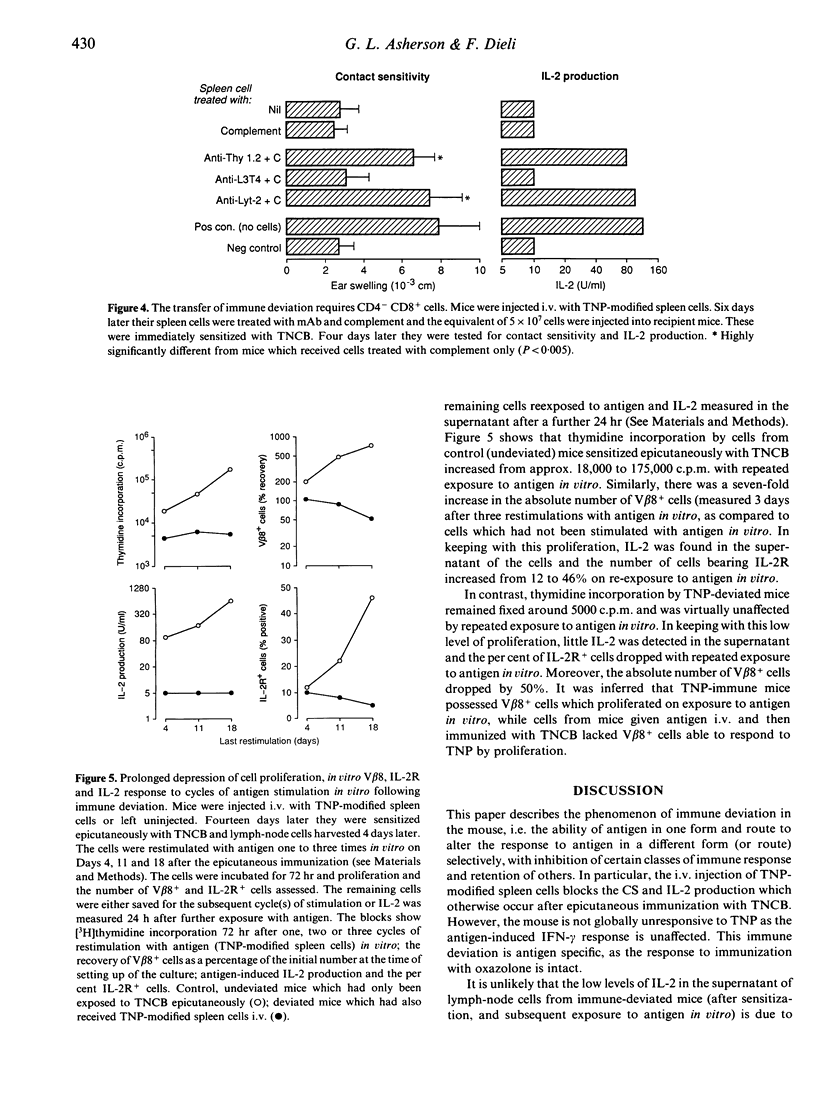
Mice were injected intravenously (i.v.) with trinitrophenyl (TNP)-modified spleen cells. They were subsequently immunized by epicutaneous application of 2,4,6-trinitrochlorobenzene (TNCB, picryl chloride) or 'oxazolone'. The intravenous injection of antigen caused immune deviation (split tolerance) with selective loss of contact sensitivity (CS) and antigen-induced interleukin-2 (IL-2) production, and concomitant retention of antigen-induced interferon-gamma (IFN-gamma) production. This phenomenon was antigen specific as the response to oxazolone was unaffected. Moreover, lymph-node cells stimulated with antigen three times in vitro (from 'deviated' mice which had been injected with antigen i.v., and then sensitized with TNCB) showed limited proliferation. The per cent of IL-2R+ cells and the absolute number of V beta 8+ cells dropped. In contrast, lymph-node cells from 'undeviated' mice showed increased proliferation and IL-2 production on repeated stimulation with antigen in vitro and the per cent of IL-2R+ cells and the absolute number of V beta 8+ cells recovered increased. Spleen cells, taken from mice 3-7 days after the injection of antigen i.v., transferred immune deviation to normal recipients i.e. following epicutaneous immunization with TNCB, the recipients showed the same selective unresponsiveness as the donors. Thy-1+ CD4- CD8+ cells were required. These findings indicate that immune deviation can be demonstrated at the level of lymphokine production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello F. B., Longo D. L., Overton R., Takacs L., Durum S. K. A role for cytokines in antigen presentation: IL-1 and IL-4 induce accessory functions of antigen-presenting cells. J Immunol. 1990 Apr 1;144(7):2572–2581. [PubMed] [Google Scholar]
- Asherson G. L., Dieli F., Gautam Y., Siew L. K., Zembala M. Major histocompatibility complex regulation of the class of the immune response: the H-2d haplotype determines poor interferon-gamma response to several antigens. Eur J Immunol. 1990 Jun;20(6):1305–1310. doi: 10.1002/eji.1830200616. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Stone S. H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: description of the phenomenon and the effect of splenectomy. Immunology. 1965 Sep;9(3):205–217. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Gautam S. C., Watkins M. C. Control of suppressor cell activity: autoanti-idiotype B cells produced by painting with picryl chloride inhibit the T-suppressor cell which blocks the efferent stage of contact sensitivity. Cell Immunol. 1982 Jun;70(1):160–169. doi: 10.1016/0008-8749(82)90141-1. [DOI] [PubMed] [Google Scholar]
- Colby W. D., Strejan G. H. Immunological tolerance of the mouse IgE system: dissociation between T cell tolerance and suppressor cell activity. Eur J Immunol. 1980 Aug;10(8):602–608. doi: 10.1002/eji.1830100806. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Davey M. J., Asherson G. L., Stone S. H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. 3. Depression of cytophilic and haemolytic antibodies by pretreatment with antigen and the effect of irradiation. Immunology. 1971 Apr;20(4):513–522. [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Greene M. I., Sugimoto M., Benacerraf B. Mechanisms of regulation of cell-mediated immune responses. I. Effect of the route of immunization with TNP-coupled syngeneic cells on the induction and suppression of contact sensitivity to picryl chloride. J Immunol. 1978 May;120(5):1604–1611. [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Sewell W., Crumpton M. J., Feldmann M., Owen M. J. Antigen-specific T cell unresponsiveness in cloned helper T cells mediated via the CD2 or CD3/Ti receptor pathways. Eur J Immunol. 1987 Nov;17(11):1641–1644. doi: 10.1002/eji.1830171118. [DOI] [PubMed] [Google Scholar]
- Loblay R. H., Fazekas de St Groth B., Pritchard-Briscoe H., Basten A. Suppressor T cell memory. II. The role of memory suppressor T cells in tolerance to human gamma globulin. J Exp Med. 1983 Mar 1;157(3):957–973. doi: 10.1084/jem.157.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar J., Asherson G. L., Colizzi V., Zembala M. IL-2 influences the balance between immunity and unresponsiveness in the picryl (TNP) contact sensitivity system by blocking the development or action of an Lyt-2+, I-J+ T suppressor cell. Cell Immunol. 1988 Nov;117(1):209–217. doi: 10.1016/0008-8749(88)90089-5. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. The induction of hapten-specific T cell tolerance using hapten-modified lymphoid membranes. II. Relative roles of suppressor T cells and clone inhibition in the tolerant state. Eur J Immunol. 1977 Mar;7(3):165–170. doi: 10.1002/eji.1830070310. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nash A. A., Ashford N. P. Split T-cell tolerance in herpes simplex virus-infected mice and its implication for anti-viral immunity. Immunology. 1982 Apr;45(4):761–767. [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J. Y. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Liew F. Y. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Rózycka D. Split unresponsiveness to the trinitrophenyl determinant. I. Manoeuvers which suppress either humoral or cell-mediated immune responses. Eur J Immunol. 1977 Dec;7(12):855–860. doi: 10.1002/eji.1830071207. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]


