Abstract
Nerve growth factor (NGF) was originally considered as a trophic factor for peripheral sympathetic and sensory neurones; however, recent reports indicate that NGF may induce proliferation of immune and haematopoietic cells. Histochemical studies conducted in human spleen and lymph nodes have suggested the presence of NGF receptor (NGF-R) immunoreactive elements in secondary follicles; however the nature of the cells bearing the NGF-R in lymphoid tissue has not been determined. In this paper we report the results of an immunohistochemical study conducted on mucosa associated lymphoid tissue. Using a specific monoclonal antibody to human NGF-R (mAb 20.4) we observed an NGF-R-immunoreactive population in all secondary lymphoid follicles examined. Double immunostaining revealed that this population was composed of follicular dendritic cells (FDC); lymphoid cells within the germinal centres did not appear to be 20.4 immunoreactive. Cell suspensions from tonsillar follicles also contained NGF-R immunopositive dendritic cells which were enriched by a 20.4 labelled magnetic bead procedure, revealing cells with the morphological characteristics of FDC. Mononuclear cells from human peripheral blood did not contain any NGF-R-immunoreactive elements using our techniques.
Full text
PDF

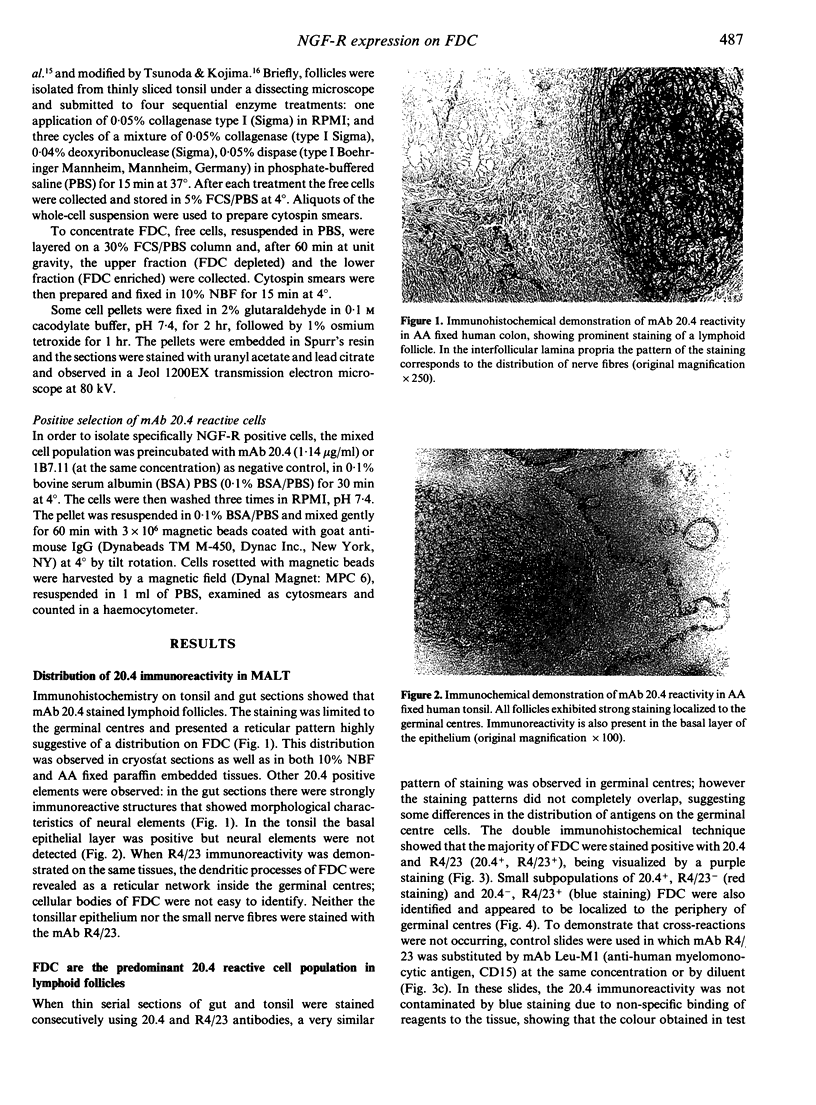
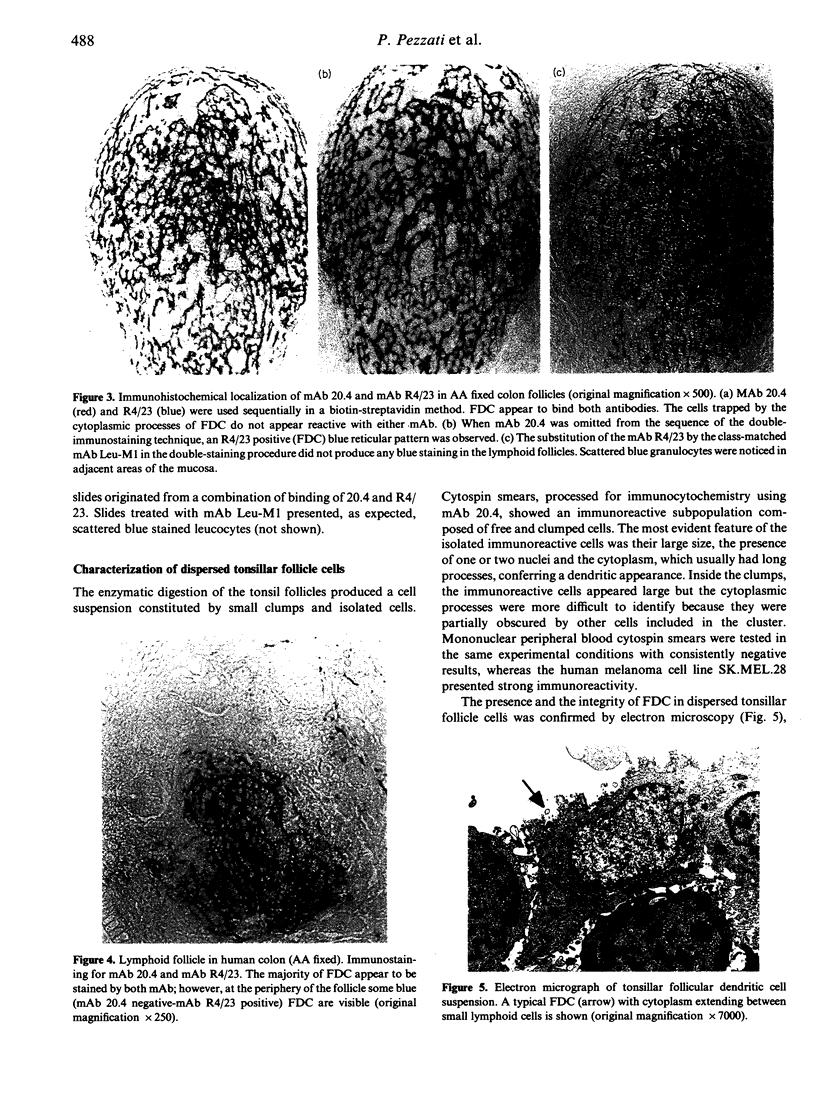


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977 Sep 16;133(2):358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Snyder S. H., Cuatrecasas P., Greene L. A. Binding of nerve growth factor receptor in sympathetic ganglia. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2519–2523. doi: 10.1073/pnas.70.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Lawman M. J., Gee A. P., Young M. Nerve growth factor: a chemotactic factor for polymorphonuclear leukocytes in vivo. J Immunol. 1985 Jan;134(1):564–568. [PubMed] [Google Scholar]
- Chesa P. G., Rettig W. J., Thomson T. M., Old L. J., Melamed M. R. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. J Histochem Cytochem. 1988 Apr;36(4):383–389. doi: 10.1177/36.4.2831267. [DOI] [PubMed] [Google Scholar]
- Gee A. P., Boyle M. D., Munger K. L., Lawman M. J., Young M. Nerve growth factor: stimulation of polymorphonuclear leukocyte chemotaxis in vitro. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7215–7218. doi: 10.1073/pnas.80.23.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen E., Cormann N., Kinet-Denoël C. The lymph follicle: a hard nut to crack. Immunol Today. 1988 Jul-Aug;9(7-8):240–243. doi: 10.1016/0167-5699(88)91223-6. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S., Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3513–3516. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilet-Leclercq C., Radoux D., Heinen E., Kinet-Denoël C., Defraigne J. O., Houben-Defresne M. P., Simar L. J. Isolation of follicular dendritic cells from human tonsils and adenoids. I. Procedure and morphological characterization. J Immunol Methods. 1984 Feb 10;66(2):235–244. doi: 10.1016/0022-1759(84)90334-x. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Stead R. H., McSharry C., Nielsen L., Bienenstock J. The role of mast cell degranulation products in mast cell hyperplasia. I. Mechanism of action of nerve growth factor. J Immunol. 1990 Mar 1;144(5):1886–1892. [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U., Ehrhard P., Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Grob P., Bothwell M., Elder D. E., Ernst C. S., Marano N., Ghrist B. F., Slemp C. C., Herlyn M., Atkinson B. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6681–6685. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal A. K., Kosco M. H., Tew J. G. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J Immunol. 1988 Jan 15;140(2):341–353. [PubMed] [Google Scholar]
- Thompson S. J., Schatteman G. C., Gown A. M., Bothwell M. A monoclonal antibody against nerve growth factor receptor. Immunohistochemical analysis of normal and neoplastic human tissue. Am J Clin Pathol. 1989 Oct;92(4):415–423. doi: 10.1093/ajcp/92.4.415. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Perez-Polo J. R. The influence of nerve growth factor on the in vitro proliferative response of rat spleen lymphocytes. J Neurosci Res. 1987;18(1):134–139. doi: 10.1002/jnr.490180120. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Werrbach-Perez K., Perez-Polo J. R. Effects of nerve growth factor on the expression of interleukin-2 receptors on cultured human lymphocytes. Ann N Y Acad Sci. 1987;496:310–311. doi: 10.1111/j.1749-6632.1987.tb35781.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda R., Kojima M. A light microscopical study of isolated follicular dendritic cell-clusters in human tonsils. Acta Pathol Jpn. 1987 Apr;37(4):575–585. doi: 10.1111/j.1440-1827.1987.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987 Jun;48(6):1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]