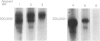Abstract
This report presents the characterization of three mouse monoclonal antibodies (mAb) reactive with the guinea-pig leucocyte common antigen (LCA); CD45 in the human nomenclature. One, IH-1, reacted with LCA on all leucocytes. The other two were more restricted: IH-2 recognized only the 220,000, 210,000 and 195,000 MW isoforms, and IH-4 the 220,000, 210,000 MW isoforms. Both IH-2 and IH-4 reacted with all B cells and all Kurloff cells [the putative guinea-pig natural killer (NK) cell]. IH-2, but not IH-4, reacted with monocytes and macrophages. Neither reacted with neutrophils. Most thymocytes expressed low levels of the IH-2 and IH-4 epitopes, with those expressing high levels located predominantly within the medulla. Most (90%) CD4+ T cells from newborn guinea-pigs expressed high levels of the IH-2 and IH-4 epitopes; this percentage decreased with age to 70% in 2-year-old animals. We have demonstrated that CD4+ T cells which express low levels of the IH-2 epitope also express low levels of the IH-4 epitope. CD8+ T cells can be divided into two subsets by IH-4 but not IH-2. The reactivities of IH-2 and IH-4 are remarkably similar to those of human anti-CD45RB and anti-CD45RA antibodies respectively. Analogies with man and other species suggest important functional differences for subpopulations of guinea-pig T lymphocytes defined by anti-CD45R antibodies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniou A. V., Parker D., Turk J. L., Tan B. T., Scheper R. J. Immunocytochemical identification and quantitation of mononuclear cells in the meninges during the development of chronic relapsing experimental allergic encephalomyelitis (CREAE) in the guinea pig. Cell Immunol. 1986 Feb;97(2):386–396. doi: 10.1016/0008-8749(86)90408-9. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Merkenschlager M., Terry L. Phenotypic diversity of the CD45 antigen and its relationship to function. Immunol Suppl. 1988;1:3–5. [PubMed] [Google Scholar]
- Debout C., Quillec M., Izard J. Natural killer activity of Kurloff cells: a direct demonstration on purified Kurloff cell suspensions. Cell Immunol. 1984 Sep;87(2):674–677. doi: 10.1016/0008-8749(84)90034-0. [DOI] [PubMed] [Google Scholar]
- Egerton M., Pruski E., Pilarski L. M. Cell generation within human thymic subsets defined by selective expression of CD45 (T200) isoforms. Hum Immunol. 1990 Apr;27(4):333–347. doi: 10.1016/0198-8859(90)90084-3. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Pilarski L. M. In situ localization of CD45 isoforms in the human thymus indicates a medullary location for the thymic generative lineage. J Immunol. 1990 Jan 1;144(1):66–74. [PubMed] [Google Scholar]
- Glennie M. J., McBride H. M., Worth A. T., Stevenson G. T. Preparation and performance of bispecific F(ab' gamma)2 antibody containing thioether-linked Fab' gamma fragments. J Immunol. 1987 Oct 1;139(7):2367–2375. [PubMed] [Google Scholar]
- Greenman J., Tutt A. L., George A. J., Pulford K. A., Stevenson G. T., Glennie M. J. Characterization of a new monoclonal anti-Fc gamma RII antibody, AT10, and its incorporation into a bispecific F(ab')2 derivative for recruitment of cytotoxic effectors. Mol Immunol. 1991 Nov;28(11):1243–1254. doi: 10.1016/0161-5890(91)90011-8. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Sharrow S. O., Johnson P., Trowbridge I. S., Hodes R. J. Expression of variable exon A-, B-, and C-specific CD45 determinants on peripheral and thymic T cell populations. J Immunol. 1992 Jan 1;148(1):19–28. [PubMed] [Google Scholar]
- Houssaint E., Tobin S., Cihak J., Lösch U. A chicken leukocyte common antigen: biochemical characterization and ontogenetic study. Eur J Immunol. 1987 Feb;17(2):287–290. doi: 10.1002/eji.1830170221. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R., McKeever D. J., Taracha E. L., Jones B. V., MacHugh N. D., Morrison W. I. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte-common antigen, CD45. Eur J Immunol. 1991 Sep;21(9):2219–2226. doi: 10.1002/eji.1830210933. [DOI] [PubMed] [Google Scholar]
- Johnson P., Greenbaum L., Bottomly K., Trowbridge I. S. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J Exp Med. 1989 Mar 1;169(3):1179–1184. doi: 10.1084/jem.169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K. A., Scollay R. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int Immunol. 1990;2(5):419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law D. A., Spruyt L. L., Paterson D. J., Williams A. F. Subsets of thymopoietic rat thymocytes defined by expression of the CD2 antigen and the MRC OX-22 determinant of the leukocyte-common antigen CD45. Eur J Immunol. 1989 Dec;19(12):2289–2295. doi: 10.1002/eji.1830191217. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightstone E. B., Marvel J. CD45RA is detected in all thymocyte subsets defined by CD4 and CD8 by using three-colour flow cytometry. Immunology. 1990 Dec;71(4):467–472. [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. A monoclonal antibody to the p220 component of sheep LCA identifies B cells and a unique lymphocyte subset. Cell Immunol. 1987 Nov;110(1):46–55. doi: 10.1016/0008-8749(87)90100-6. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Fisher A. G. CD45 isoform switching precedes the activation-driven death of human thymocytes by apoptosis. Int Immunol. 1991 Jan;3(1):1–7. doi: 10.1093/intimm/3.1.1. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Tyrosine phosphorylation in T-cell activation. Scand J Immunol. 1991 Sep;34(3):259–264. doi: 10.1111/j.1365-3083.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M., Gillitzer R., Zola H., Shortman K., Scollay R. Definition of the thymic generative lineage by selective expression of high molecular weight isoforms of CD45 (T200). Eur J Immunol. 1989 Apr;19(4):589–597. doi: 10.1002/eji.1830190403. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. Subsets of rat CD4+ T cells defined by their differential expression of variants of the CD45 antigen: developmental relationships and in vitro and in vivo functions. Curr Top Microbiol Immunol. 1990;159:79–96. doi: 10.1007/978-3-642-75244-5_5. [DOI] [PubMed] [Google Scholar]
- Pulido R., Cebrián M., Acevedo A., de Landázuri M. O., Sánchez-Madrid F. Comparative biochemical and tissue distribution study of four distinct CD45 antigen specificities. J Immunol. 1988 Jun 1;140(11):3851–3857. [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sarmiento U. M., Valli V. E. A canine lymphocyte surface antigen detectable by a monoclonal antibody (DT200). Can J Vet Res. 1987 Jan;51(1):110–116. [PMC free article] [PubMed] [Google Scholar]
- Schäfer H., Baker D., Thiele B., Burger R. Structure, cellular distribution, and functional characteristics of the guinea pig leucocyte common antigen. Cell Immunol. 1990 Jul;128(2):370–384. doi: 10.1016/0008-8749(90)90034-o. [DOI] [PubMed] [Google Scholar]
- Stevenson F. K., Glennie M. J., Johnston D. M., Tutt A. L., Stevenson G. T. Consumption of monoclonal anti-idiotypic antibody by neoplastic B lymphocytes: a guide for immunotherapy. Br J Cancer. 1984 Sep;50(3):407–413. doi: 10.1038/bjc.1984.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Eady R. P., Hough D. W., Jurd R. D., Stevenson F. K. Surface immunoglobulin of guinea-pig leukaemic lymphocytes. Immunology. 1975 May;28(5):807–820. [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tighe H., Clark M., Waldmann H. Blocking of cytotoxic T cell function by monoclonal antibodies against the CD45 antigen (T200/leukocyte-common antigen). Transplantation. 1987 Dec;44(6):818–823. doi: 10.1097/00007890-198712000-00020. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Ostergaard H. L., Johnson P. CD45: a leukocyte-specific member of the protein tyrosine phosphatase family. Biochim Biophys Acta. 1991 Oct 16;1095(1):46–56. doi: 10.1016/0167-4889(91)90043-w. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]