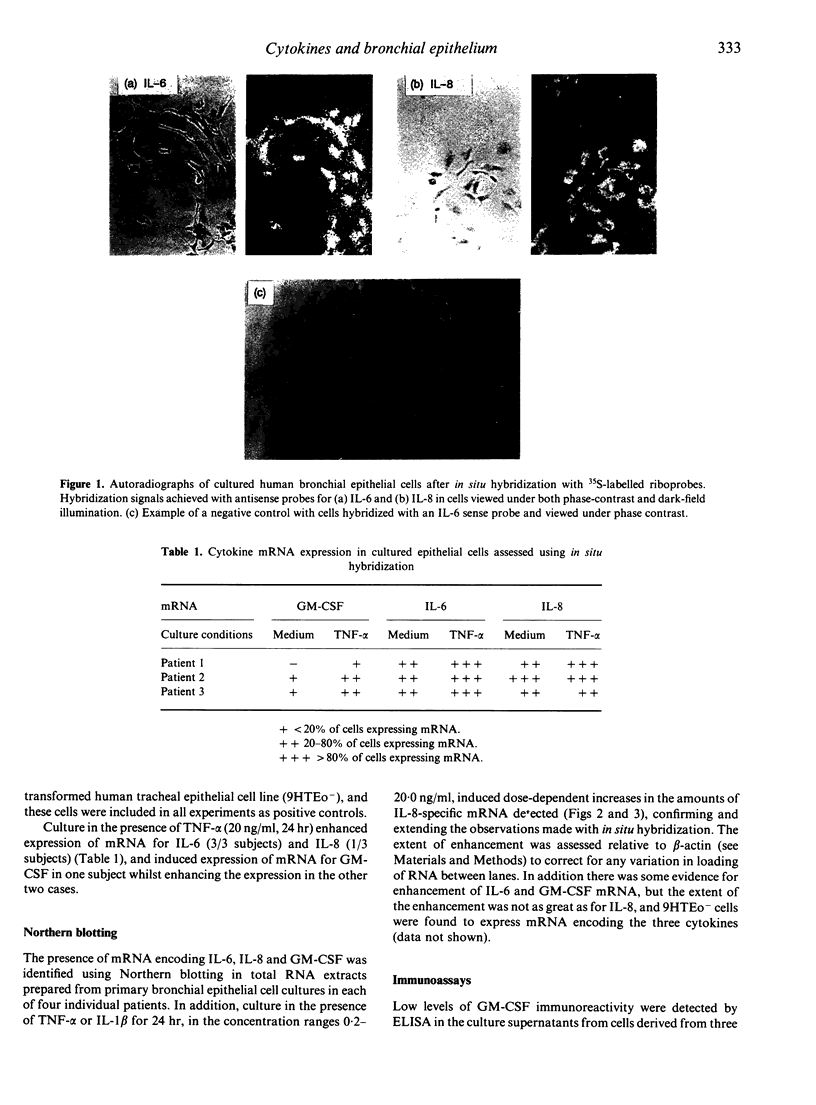
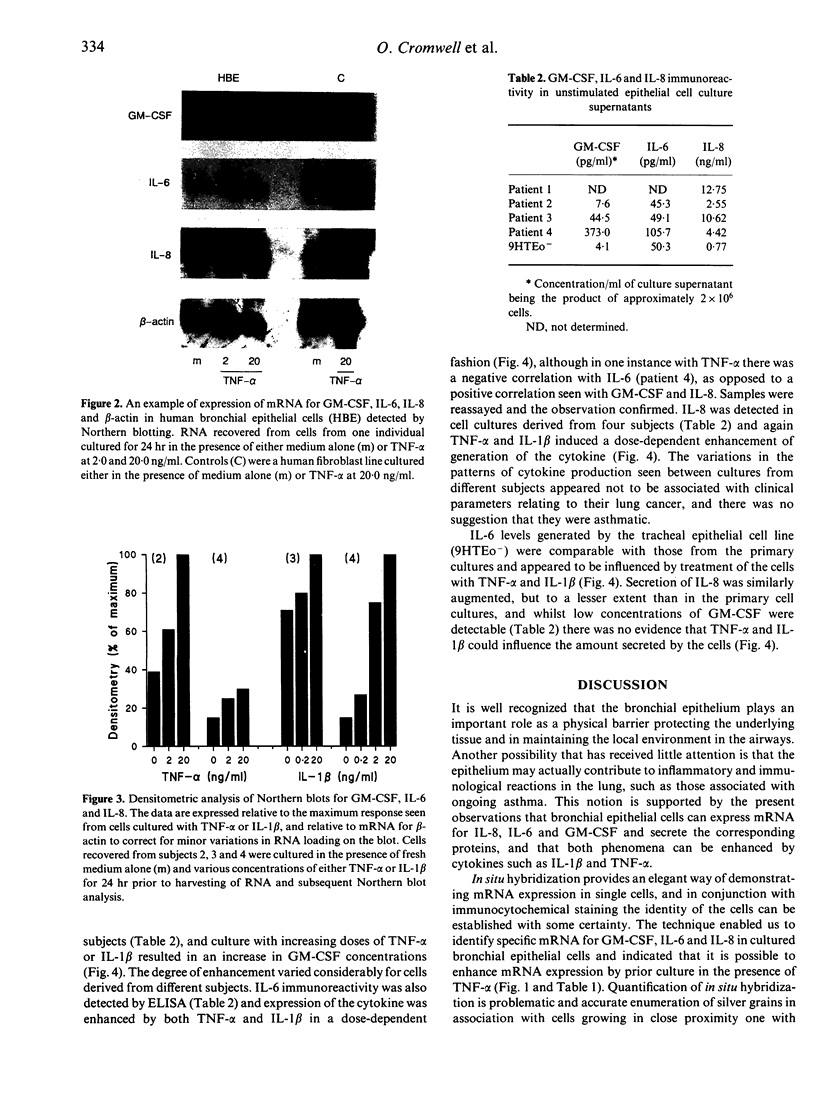
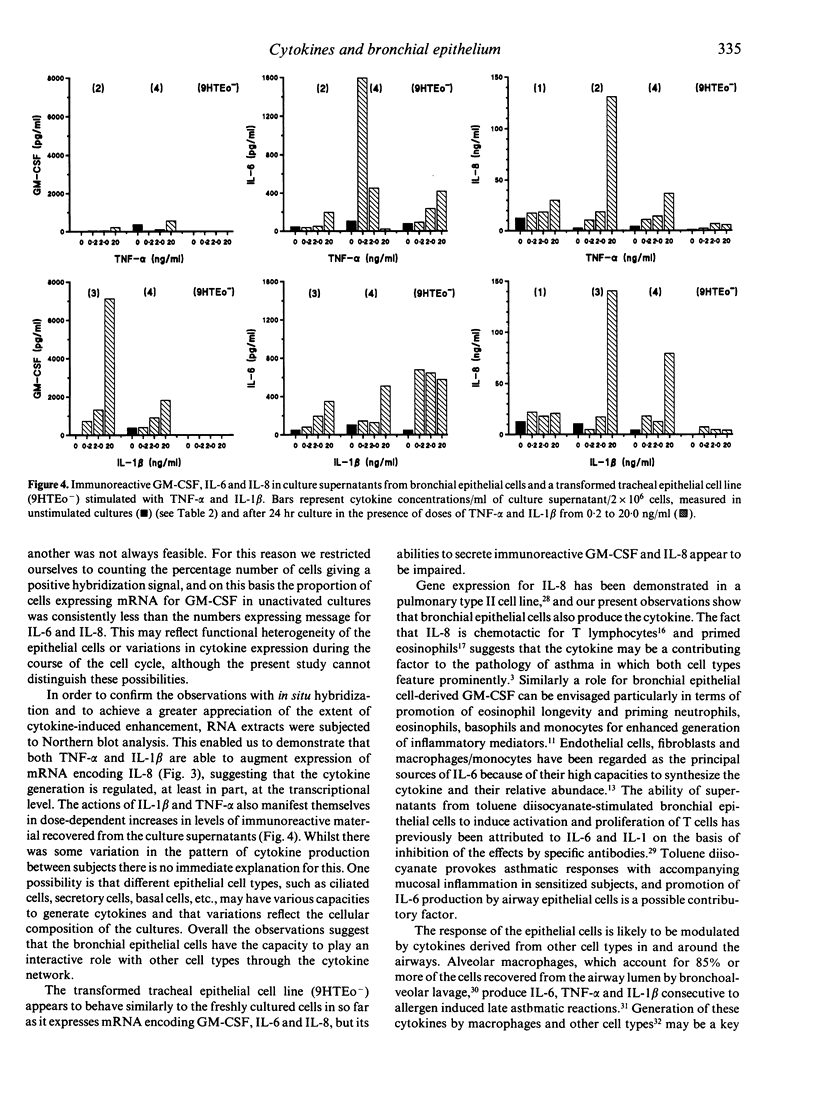
Abstract
We have tested the hypothesis that the bronchial epithelium has the capacity to generate and release cytokines that could contribute to inflammatory events associated with inflammatory lung diseases. Messenger RNA (mRNA) for interleukin-6 (IL-6), IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) was identified in human bronchial epithelial cell primary cultures, characterized on the basis of staining for cytokeratin, using both in situ hybridization and Northern blotting. Using in situ hybridization we have shown that the majority of the cells expressed mRNA for IL-6 and IL-8, whereas fewer than 20% of cells expressed message for GM-CSF. The numbers of cells expressing message were increased by culture with tumour necrosis factor-alpha (TNF-alpha) (20 ng/ml, 24 hr). These observations were substantiated by Northern blotting, which showed that both TNF-alpha and IL-1 beta were able to induce a dose-dependent increase in IL-8-specific mRNA. Immunoreactive IL-6 and GM-CSF were detected and quantified in the culture supernatants by ELISA, and IL-8 by radioimmunoassay. The levels of immunoreactivity were increased by incubation of epithelial cells with either IL-1 beta or TNF-alpha for 24 hr. A transformed tracheal epithelial cell line (9HTEo-) expressed mRNA for IL-6, IL-8 and GM-CSF but, whereas levels of immunoreactive IL-6 in culture supernatants were comparable with those in primary cell cultures, levels of IL-8 were low and GM-CSF trivial. These observations indicate that the bronchial epithelium has the potential to be a major source of IL-8 and a number of other cytokines, and that production can be amplified substantially by IL-1 beta and TNF-alpha. The bronchial epithelium is ideally situated to modulate inflammatory and immunological events in and around the airways, and these observations suggest that it could contribute to promote and sustain inflammatory and immunological processes in inflammatory lung diseases such asthma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi M., Saito M., Koeffler H. P. Lymphotoxin: stimulation and regulation of colony-stimulating factors in fibroblasts. Blood. 1989 Nov 15;74(7):2383–2390. [PubMed] [Google Scholar]
- Barnes P. J. Asthma as an axon reflex. Lancet. 1986 Feb 1;1(8475):242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J., Sheller J. R., Nadel J. A. Bronchial hyperreactivity. Am Rev Respir Dis. 1980 Feb;121(2):389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Churchill L., Friedman B., Schleimer R. P., Proud D. Production of granulocyte-macrophage colony-stimulating factor by cultured human tracheal epithelial cells. Immunology. 1992 Jan;75(1):189–195. [PMC free article] [PubMed] [Google Scholar]
- Collins P. D., Jose P. J., Williams T. J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991 Jan 15;146(2):677–684. [PubMed] [Google Scholar]
- Cox G., Ohtoshi T., Vancheri C., Denburg J. A., Dolovich J., Gauldie J., Jordana M. Promotion of eosinophil survival by human bronchial epithelial cells and its modulation by steroids. Am J Respir Cell Mol Biol. 1991 Jun;4(6):525–531. doi: 10.1165/ajrcmb/4.6.525. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Welsh M. J., Li M., Finkbeiner W. E., Nadel J. A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Wharton J., Terenghi G., Hassall C. J., Aimi J., Taylor K. M., Nakazato H., Dixon J. E., Burnstock G., Polak J. M. Localization of atrial natriuretic peptide mRNA and immunoreactivity in the rat heart and human atrial appendage. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6760–6764. doi: 10.1073/pnas.84.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Wardlaw A. J., Nelson F. C., Collins J. V., Kay A. B. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989 Dec;140(6):1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Kay A. B. Asthma and inflammation. J Allergy Clin Immunol. 1991 May;87(5):893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am J Respir Cell Mol Biol. 1990 Jun;2(6):479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- Marini M., Soloperto M., Mezzetti M., Fasoli A., Mattoli S. Interleukin-1 binds to specific receptors on human bronchial epithelial cells and upregulates granulocyte/macrophage colony-stimulating factor synthesis and release. Am J Respir Cell Mol Biol. 1991 Jun;4(6):519–524. doi: 10.1165/ajrcmb/4.6.519. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y. Bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jan;135(1):250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- Ruef C., Coleman D. L. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990 Jan-Feb;12(1):41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- Salari H., Chan-Yeung M. Release of 15-hydroxyeicosatetraenoic acid (15-HETE) and prostaglandin E2 (PGE2) by cultured human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1989 Sep;1(3):245–250. doi: 10.1165/ajrcmb/1.3.245. [DOI] [PubMed] [Google Scholar]
- Smith W. B., Gamble J. R., Clark-Lewis I., Vadas M. A. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991 Jan;72(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- Soloperto M., Mattoso V. L., Fasoli A., Mattoli S. A bronchial epithelial cell-derived factor in asthma that promotes eosinophil activation and survival as GM-CSF. Am J Physiol. 1991 Jun;260(6 Pt 1):L530–L538. doi: 10.1152/ajplung.1991.260.6.L530. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M. Epithelium-derived relaxing factor(s) and bronchial reactivity. Am Rev Respir Dis. 1988 Dec;138(6 Pt 2):S24–S30. doi: 10.1164/ajrccm/138.6_Pt_2.S24. [DOI] [PubMed] [Google Scholar]
- Warringa R. A., Koenderman L., Kok P. T., Kreukniet J., Bruijnzeel P. L. Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood. 1991 Jun 15;77(12):2694–2700. [PubMed] [Google Scholar]
- Widdicombe J. H., Coleman D. L., Finkbeiner W. E., Tuet I. K. Electrical properties of monolayers cultured from cells of human tracheal mucosa. J Appl Physiol (1985) 1985 May;58(5):1729–1735. doi: 10.1152/jappl.1985.58.5.1729. [DOI] [PubMed] [Google Scholar]