Abstract
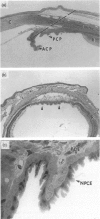
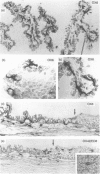
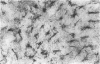
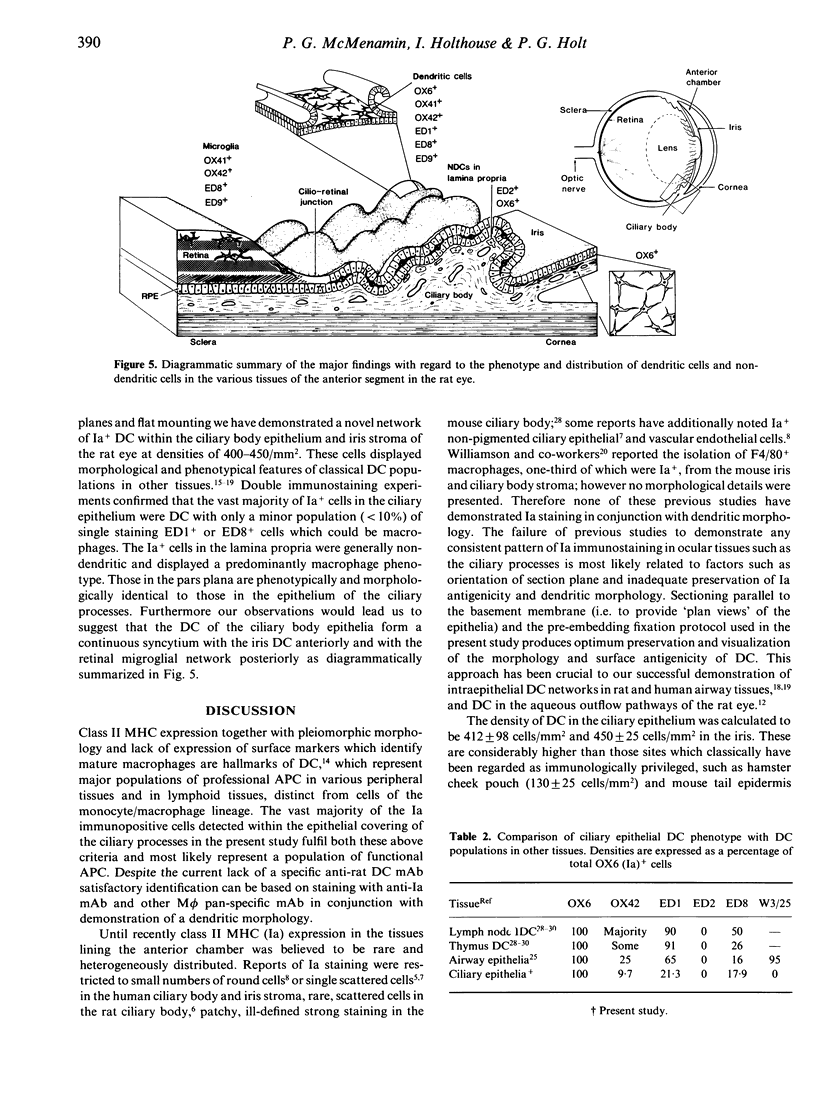
The density, distribution and surface phenotype of dendritic cells (DC) and macrophage populations within the ciliary body and iris of Wistar Furth rats were studied by a combination of flat mounting, tangential sectioning, pre-embedding fixation, with a single and double immunohistochemical techniques. Monoclonal antibodies included anti-Ia (OX6) and other dendritic cell/macrophage (ED1 and ED8) or mature tissue macrophage markers (ED2). Single and double staining revealed a network (approximately 400 cells/mm2) of Ia+ cells within the epithelium of the ciliary processes with the morphological and surface phenotypic characteristics of DC populations in other tissues. A minor proportion of DC co-expressed ED1 and ED8, but not ED2. In contrast the immunopositive cells in the lamina propria displayed a more generalized phenotype, including ED2 expression, and pleiomorphic morphology suggesting a preponderance of cells of macrophage lineage. Flat mounts of iris revealed a remarkably regular network of Ia+ DC at a density of 450 cells/mm2. The network of DC in the ciliary epithelium terminated at the cilioretinal junction where they formed a continuous syncytium with retinal microglia which did not display Ia staining. The demonstration of networks of cells with relevant morphological and phenotypical properties of professional antigen-presenting cells at strategic locations within the eye has important implications in relation to ocular immune regulation and on the theories of the mechanism of anterior chamber-associated immune deviation (ACAID). Namely, until now it has been assumed that 'immune privilege' in the anterior chamber of the eye is partly a consequence of there being a paucity of class II+ cells in the surrounding tissues. Dendritic cells in the eye may function as antigen-presenting cells, sampling endogenous and exogenous intraocular antigens and possibly migrating from the eye to draining lymphoid organs (the spleen) where they may generate systemic immune responses. Equally dendritic cells could potentially regulate local immune responses for example in various forms of autoimmune uveoretinal inflammatory disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Zhang Y. W. Species differences in the effects of endothelin-1 on myo-inositol trisphosphate accumulation, cyclic AMP formation and contraction of isolated iris sphincter of rabbit and other species. Invest Ophthalmol Vis Sci. 1991 Jul;32(8):2432–2438. [PubMed] [Google Scholar]
- Ahlfors E. E., Larsson P. A., Bergstresser P. R. Langerhans cell surface densities in rat oral mucosa and human buccal mucosa. J Oral Pathol. 1985 May;14(5):390–397. doi: 10.1111/j.1600-0714.1985.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Bakker M., Kijlstra A. The expression of HLA-antigens in the human anterior uvea. Curr Eye Res. 1985 May;4(5):599–604. doi: 10.3109/02713688508999991. [DOI] [PubMed] [Google Scholar]
- Baudouin C., Fredj-Reygrobellet D., Gastaud P., Lapalus P. HLA DR and DQ distribution in normal human ocular structures. Curr Eye Res. 1988 Sep;7(9):903–911. doi: 10.3109/02713688808997247. [DOI] [PubMed] [Google Scholar]
- Benson J. L., Niederkorn J. Y. The presence of donor-derived class II-positive cells abolishes immune privilege in the anterior chamber of the eye. Transplantation. 1991 Apr;51(4):834–838. doi: 10.1097/00007890-199104000-00018. [DOI] [PubMed] [Google Scholar]
- Bergstresser P. R., Fletcher C. R., Streilein J. W. Surface densities of Langerhans cells in relation to rodent epidermal sites with special immunologic properties. J Invest Dermatol. 1980 Feb;74(2):77–80. doi: 10.1111/1523-1747.ep12519909. [DOI] [PubMed] [Google Scholar]
- Claassen E., Adler L. T., Adler F. L. Double immunocytochemical staining for the in situ study of allotype distribution during an anti-trinitrophenyl immune response in chimeric rabbits. J Histochem Cytochem. 1986 Aug;34(8):989–994. doi: 10.1177/34.8.2426338. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. G., Döpp E. A., Neefjes J. J., Beelen R. H., Dijkstra C. D. Heterogeneity of macrophages in the rat evidenced by variability in determinants: two new anti-rat macrophage antibodies against a heterodimer of 160 and 95 kd (CD11/CD18). J Leukoc Biol. 1989 Dec;46(6):556–564. doi: 10.1002/jlb.46.6.556. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Gillette T. E., Chandler J. W., Greiner J. V. Langerhans cells of the ocular surface. Ophthalmology. 1982 Jun;89(6):700–711. doi: 10.1016/s0161-6420(82)34737-5. [DOI] [PubMed] [Google Scholar]
- Hamel C. P., Detrick B., Hooks J. J. Evaluation of Ia expression in rat ocular tissues following inoculation with interferon-gamma. Exp Eye Res. 1990 Feb;50(2):173–182. doi: 10.1016/0014-4835(90)90228-m. [DOI] [PubMed] [Google Scholar]
- Helbig H., Gurley R. C., Reichl R. J., Mahdi R., Nussenblatt R. B., Palestine A. G. Induction of MHC class II antigen in cultured bovine ciliary epithelial cells. Graefes Arch Clin Exp Ophthalmol. 1990;228(6):556–561. doi: 10.1007/BF00918490. [DOI] [PubMed] [Google Scholar]
- Hoekzema R., Verhagen C., van Haren M., Kijlstra A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):532–539. [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A., Phillips M. J., McMenamin P. G. Ia-positive dendritic cells form a tightly meshed network within the human airway epithelium. Clin Exp Allergy. 1989 Nov;19(6):597–601. doi: 10.1111/j.1365-2222.1989.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Brain macrophages: questions of origin and interrelationship. Brain Res. 1988 Apr-Jun;472(2):165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Latina M., Flotte T., Crean E., Sherwood M. E., Granstein R. D. Immunohistochemical staining of the human anterior segment. Evidence that resident cells play a role in immunologic responses. Arch Ophthalmol. 1988 Jan;106(1):95–99. doi: 10.1001/archopht.1988.01060130101037. [DOI] [PubMed] [Google Scholar]
- Loeffler K. U., McMenamin P. G. Evaluation of subretinal macrophage-like cells in the human fetal eye. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1628–1636. [PubMed] [Google Scholar]
- MacPherson G. G., Fossum S., Harrison B. Properties of lymph-borne (veiled) dendritic cells in culture. II. Expression of the IL-2 receptor: role of GM-CSF. Immunology. 1989 Sep;68(1):108–113. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Loeffler K. U. Cells resembling intraventricular macrophages are present in the subretinal space of human foetal eyes. Anat Rec. 1990 Jun;227(2):245–253. doi: 10.1002/ar.1092270213. [DOI] [PubMed] [Google Scholar]
- Niederkorn J. Y., Streilein J. W. Induction of anterior chamber-associated immune deviation (ACAID) by allogeneic intraocular tumors does not require splenic metastases. J Immunol. 1982 Jun;128(6):2470–2474. [PubMed] [Google Scholar]
- Okumura A., Mochizuki M., Nishi M., Herbort C. P. Endotoxin-induced uveitis (EIU) in the rat: a study of inflammatory and immunological mechanisms. Int Ophthalmol. 1990 Jan;14(1):31–36. doi: 10.1007/BF00131166. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Modulation of CD4 antigen on macrophages and microglia in rat brain. J Exp Med. 1987 Oct 1;166(4):1138–1143. doi: 10.1084/jem.166.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Rowden G. Expression of Ia antigens on Langerhans cells in mice, guinea pigs, and man. J Invest Dermatol. 1980 Jul;75(1):22–31. doi: 10.1111/1523-1747.ep12521071. [DOI] [PubMed] [Google Scholar]
- Schon-Hegrad M. A., Oliver J., McMenamin P. G., Holt P. G. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991 Jun 1;173(6):1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Schwender S., Imrich H., Dörries R., Butcher G. W., ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Cousins S. W. Aqueous humor factors and their effect on the immune response in the anterior chamber. Curr Eye Res. 1990;9 (Suppl):175–182. doi: 10.3109/02713689008999439. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Niederkorn J. Y. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981 May 1;153(5):1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompsett E., Abi-Hanna D., Wakefield D. Immunological privilege in the eye: a review. Curr Eye Res. 1990 Dec;9(12):1141–1145. doi: 10.3109/02713689009003470. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Kaplan H. J., Chan W. C., Johnson M. The distribution and ontogeny of MHC antigens in murine ocular tissue. Invest Ophthalmol Vis Sci. 1987 Aug;28(8):1383–1389. [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1. Evidence that an antigen-specific, ACAID-inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991 Apr 15;146(8):2610–2617. [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1. Evidence that an antigen-specific, ACAID-inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991 Apr 15;146(8):2610–2617. [PubMed] [Google Scholar]
- Williamson J. S., Bradley D., Streilein J. W. Immunoregulatory properties of bone marrow-derived cells in the iris and ciliary body. Immunology. 1989 May;67(1):96–102. [PMC free article] [PubMed] [Google Scholar]
- Williamson J. S., DiMarco S., Streilein J. W. Immunobiology of Langerhans cells on the ocular surface. I. Langerhans cells within the central cornea interfere with induction of anterior chamber associated immune deviation. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1527–1532. [PubMed] [Google Scholar]
- Williamson J. S., Streilein J. W. Induction of delayed hypersensitivity to alloantigens coinjected with Langerhans cells into the anterior chamber of the eye. Abrogation of anterior chamber-associated immune deviation. Transplantation. 1989 Mar;47(3):519–524. doi: 10.1097/00007890-198903000-00024. [DOI] [PubMed] [Google Scholar]