Abstract
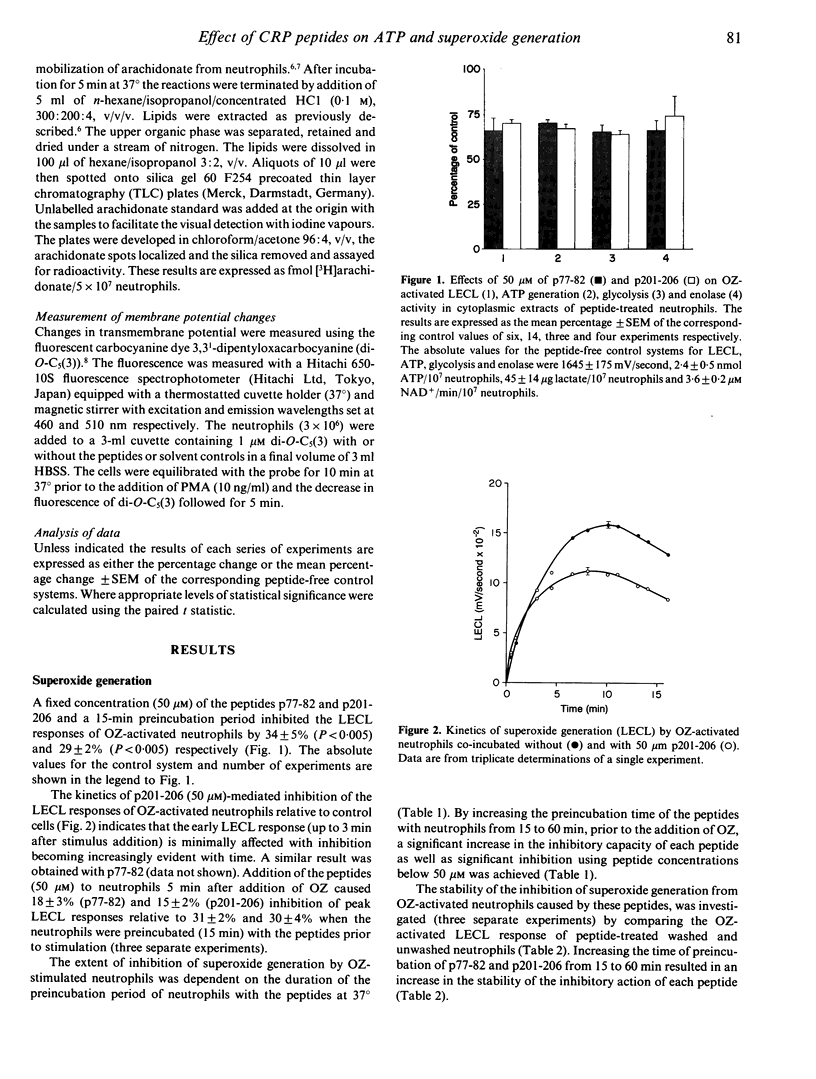
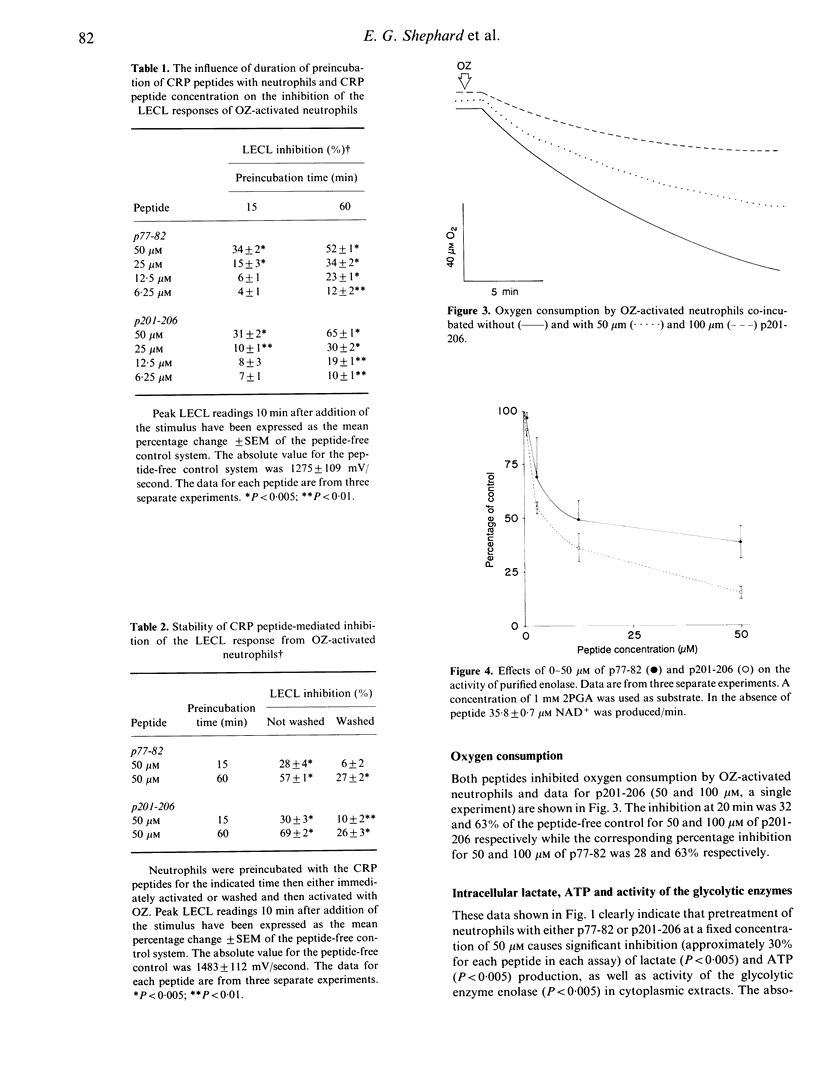
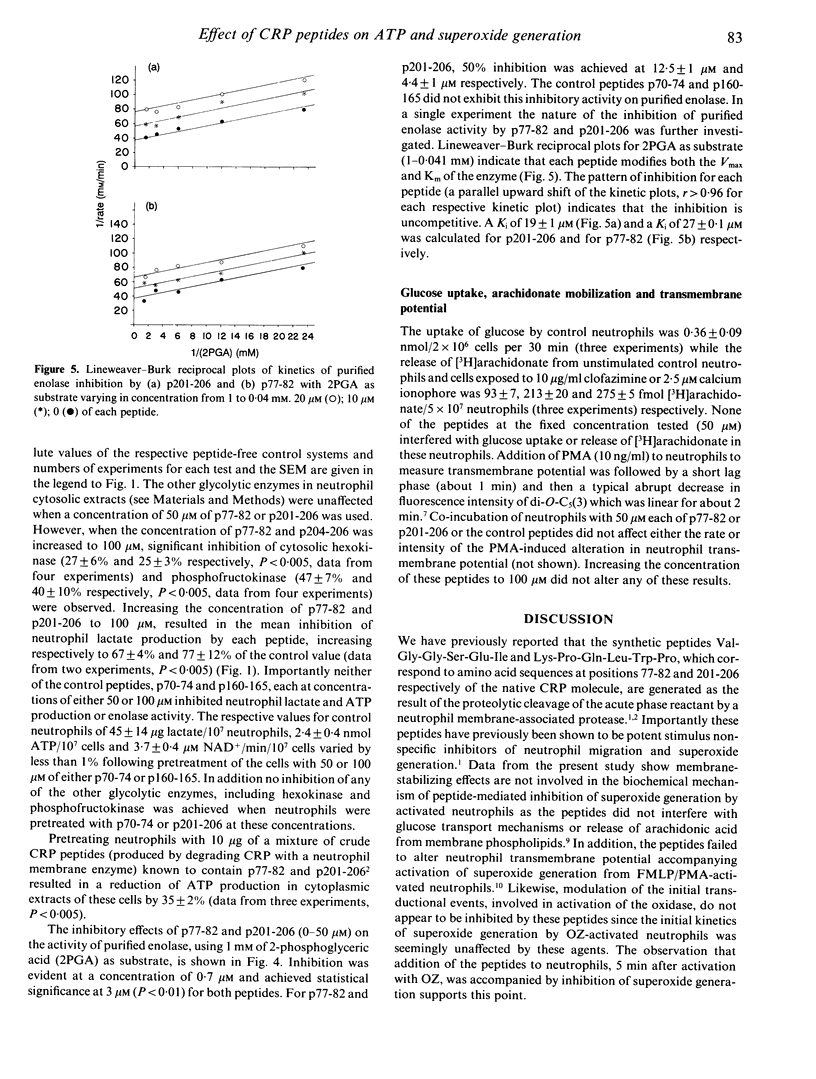
The nature and the biochemical mechanism of inhibition of neutrophil membrane-associated oxidative metabolism by two synthetic peptides p77-82 and p201-206 (amino acid sequences Val-Gly-Gly-Ser-Glu-Ile and Lys-Pro-Gln-Leu-Trp-Pro respectively, from the primary amino acid sequence of C-reactive protein) have been ascertained. Preincubating neutrophils for 15 min with 50 microM of p77-82 or p201-206 resulted in superoxide generation by opsonized zymosan stimulated neutrophils being inhibited by 34 +/- 2% (P less than 0.005) and 29 +/- 2% (P less than 0.005) respectively. With a 60-min preincubation period 6.25 microM of p77-82 or p201-206 was effective in inhibiting this superoxide generation by 12 +/- 2% (P less than 0.01) and 10 +/- 1% (P less than 0.01) respectively. Neither peptide inhibited neutrophil arachidonic acid release, transmembrane potential or transductional events preceding superoxide generation. Inhibition of neutrophil functions was found to be due to the ability of each peptide (50 microM) following a 15-min preincubation period to inhibit both neutrophil glycolysis and ATP generation by approximately 30%. The inhibition of ATP generation and glycolysis in neutrophils is attributable to the ability of these peptides to inhibit uncompetitively the glycolytic enzyme enolase. Using purified enolase the relative Ki values for p77-82 and p201-206 were 27 and 19 microM respectively. Inhibition of neutrophil function by the peptides is concluded to be due to effective interference of neutrophil energy metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Beyers A. D., Savage J. E., Nel A. E. Apparent involvement of phospholipase A2, but not protein kinase C, in the pro-oxidative interactions of clofazimine with human phagocytes. Biochem Pharmacol. 1988 Dec 15;37(24):4635–4641. doi: 10.1016/0006-2952(88)90332-2. [DOI] [PubMed] [Google Scholar]
- Buchta R., Gennaro R., Pontet M., Fridkin M., Romeo D. C-reactive protein decreases protein phosphorylation in stimulated human neutrophils. FEBS Lett. 1988 Sep 12;237(1-2):173–177. doi: 10.1016/0014-5793(88)80195-9. [DOI] [PubMed] [Google Scholar]
- Diplock A. T., Lucy J. A., Verrinder M., Zieleniewski A. alpha-Tocopherol and the permeability to glucose and chromate of unsaturated liposomes. FEBS Lett. 1977 Oct 15;82(2):341–344. doi: 10.1016/0014-5793(77)80616-9. [DOI] [PubMed] [Google Scholar]
- Fridkin M., Najjar V. A. Tuftsin: its chemistry, biology, and clinical potential. Crit Rev Biochem Mol Biol. 1989;24(1):1–40. doi: 10.3109/10409238909082550. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Structural determinants of the eosinophil: chemotactic activity of the acidic tetrapeptides of eosinophil chemotactic factor of anaphylaxis. J Exp Med. 1976 Dec 1;144(6):1424–1437. doi: 10.1084/jem.144.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Continuous phosphorylation of both the 47 and the 49 kDa proteins occurs during superoxide production by neutrophils. Biochim Biophys Acta. 1990 May 2;1052(2):299–305. doi: 10.1016/0167-4889(90)90225-3. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Storm E., Day H. J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972 Apr;46(2):489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- KUSHNER I., RAKITA L., KAPLAN M. H. Studies of acute-phase protein. II. Localization of Cx-reactive protein in heart in induced myocardial infarction in rabbits. J Clin Invest. 1963 Feb;42:286–292. doi: 10.1172/JCI104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Ohta M., Nojiri H., Kakinuma K., Saito M., Takaku F., Miura Y. Functional maturation of membrane potential changes and superoxide-producing capacity during differentiation of human granulocytes. J Clin Invest. 1984 Apr;73(4):1062–1071. doi: 10.1172/JCI111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkenberg I., Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 1984 Jun 8;71(1):61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E. G., Anderson R., Rosen O., Myer M. S., Fridkin M., Strachan A. F., De Beer F. C. Peptides generated from C-reactive protein by a neutrophil membrane protease. Amino acid sequence and effects of peptides on neutrophil oxidative metabolism and chemotaxis. J Immunol. 1990 Sep 1;145(5):1469–1476. [PubMed] [Google Scholar]
- Shephard E. G., Beer S. M., Anderson R., Strachan A. F., Nel A. E., de Beer F. C. Generation of biologically active C-reactive protein peptides by a neutral protease on the membrane of phorbol myristate acetate-stimulated neutrophils. J Immunol. 1989 Nov 1;143(9):2974–2981. [PubMed] [Google Scholar]
- Simchowitz L., Mehta J., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979 Jul;22(7):755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Tao W., Molski T. F., Naccache P. H., Sha'afi R. I. Leukotriene B4 mobilizes calcium without the breakdown of polyphosphoinositides and the production of phosphatidic acid in rabbit neutrophils. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5966–5969. doi: 10.1073/pnas.81.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]


