Abstract
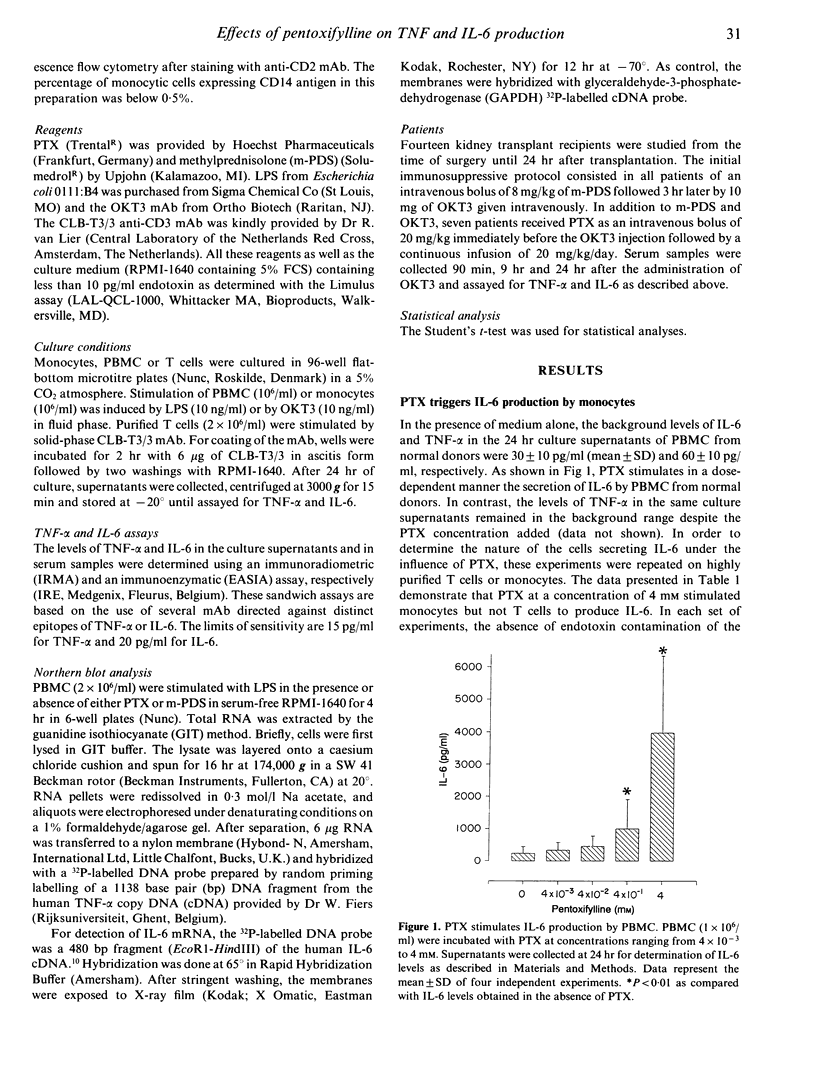
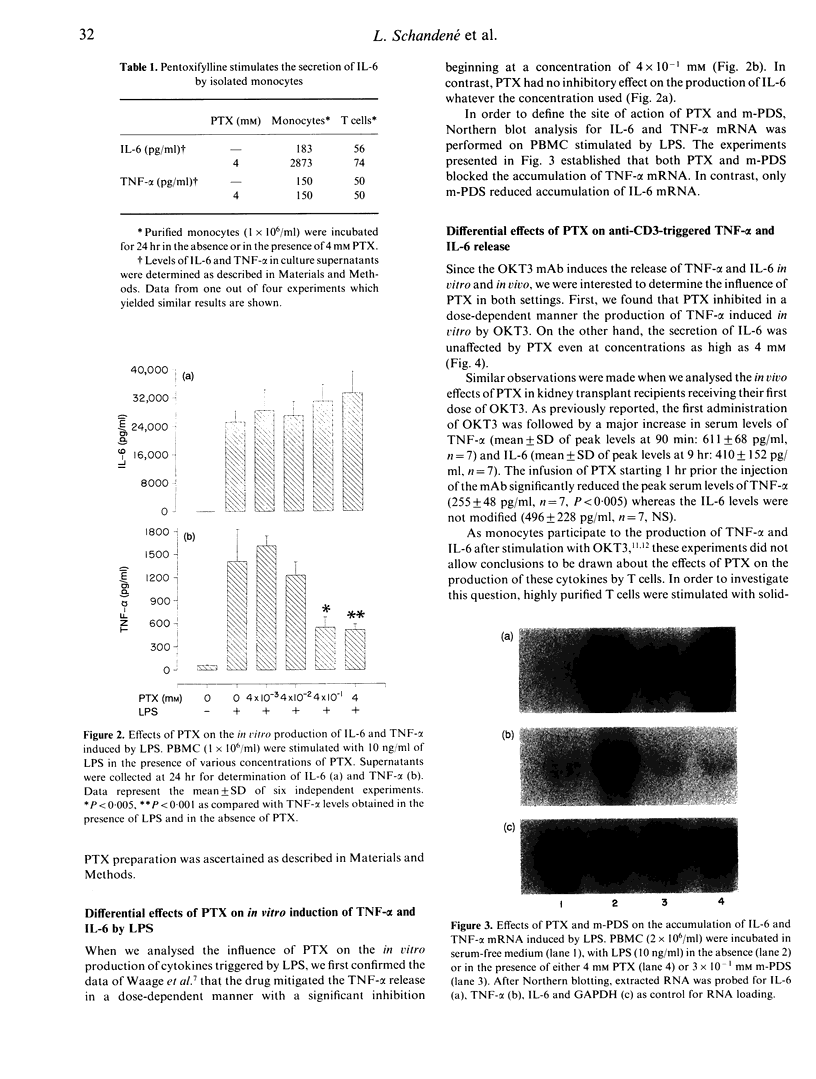
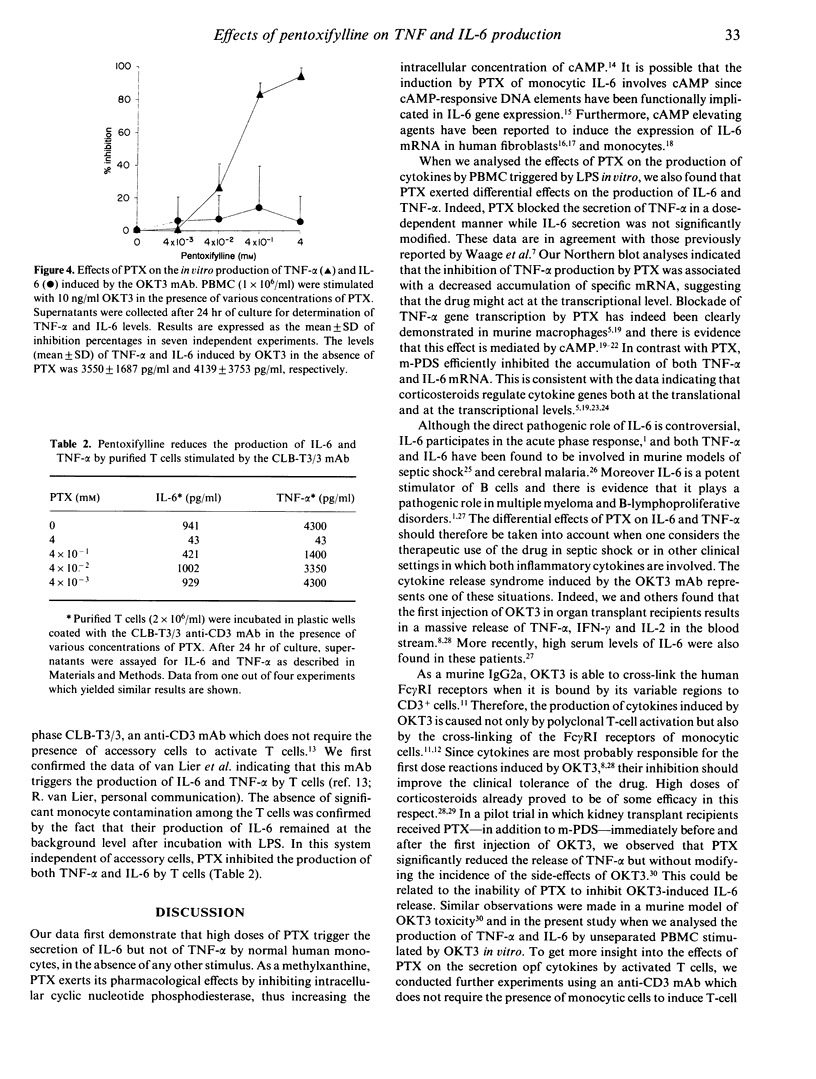
Pentoxifylline (PTX) is a methylxanthine compound known to inhibit the production of tumour necrosis factor-alpha (TNF-alpha) by monocytic cells. In this study, we found that PTX differentially regulates the production of TNF-alpha and interleukin-6 (IL-6). Indeed, PTX at high concentrations triggers the production of IL-6 but not of TNF-alpha by peripheral blood mononuclear cells (PBMC). Further experiments indicated that monocytes are responsible for this PTX-induced IL-6 production. When PBMC were stimulated with LPS, PTX was found to inhibit the secretion of TNF-alpha as well as the accumulation of TNF-alpha messenger RNA (mRNA). In contrast, no inhibitory effect was observed on the induction of IL-6. Similar results were obtained when PBMC were stimulated with OKT3 monoclonal antibody (mAb). In addition, the in vivo administration of PTX in transplant patients receiving the first dose of OKT3 allowed to decrease the systemic release of TNF-alpha but not of IL-6. Since monocytes represent a major source of TNF-alpha and IL-6 in these settings, additional experiments were performed in vitro on purified T cells stimulated with the CLB-T3/3, an anti-CD3 mAb which does not require the presence of accessory cells to activate T cells. In this system, PTX was found to inhibit the secretion of both TNF-alpha and IL-6 by T cells. We suggest that cAMP could be involved in these differential effects of PTX on production of TNF-alpha and of IL-6.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Schandene L., Goldman M., Crusiaux A., Vereerstraeten P., De Pauw L., Wybran J., Kinnaert P., Dupont E., Toussaint C. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation. 1989 Apr;47(4):606–608. doi: 10.1097/00007890-198904000-00008. [DOI] [PubMed] [Google Scholar]
- Alegre M. L., Gastaldello K., Abramowicz D., Kinnaert P., Vereerstraeten P., De Pauw L., Vandenabeele P., Moser M., Leo O., Goldman M. Evidence that pentoxifylline reduces anti-CD3 monoclonal antibody-induced cytokine release syndrome. Transplantation. 1991 Oct;52(4):674–679. doi: 10.1097/00007890-199110000-00018. [DOI] [PubMed] [Google Scholar]
- Bessler H., Gilgal R., Djaldetti M., Zahavi I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 1986 Dec;40(6):747–754. doi: 10.1002/jlb.40.6.747. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988 Aug 15;141(4):1197–1201. [PubMed] [Google Scholar]
- Doherty G. M., Jensen J. C., Alexander H. R., Buresh C. M., Norton J. A. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991 Aug;110(2):192–198. [PubMed] [Google Scholar]
- Endres S., Fülle H. J., Sinha B., Stoll D., Dinarello C. A., Gerzer R., Weber P. C. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology. 1991 Jan;72(1):56–60. [PMC free article] [PubMed] [Google Scholar]
- Goldman M., Abramowicz D., De Pauw L., Alegre M. L., Widera I., Vereerstraeten P., Kinnaert OKT3-induced cytokine release attenuation by high-dose methylprednisolone. Lancet. 1989 Sep 30;2(8666):802–803. doi: 10.1016/s0140-6736(89)90864-7. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Han J., Huez G., Beutler B. Interactive effects of the tumor necrosis factor promoter and 3'-untranslated regions. J Immunol. 1991 Mar 15;146(6):1843–1848. [PubMed] [Google Scholar]
- Han J., Thompson P., Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990 Jul 1;172(1):391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Krutmann J., Kirnbauer R., Köck A., Schwarz T., Schöpf E., May L. T., Sehgal P. B., Luger T. A. Cross-linking Fc receptors on monocytes triggers IL-6 production. Role in anti-CD3-induced T cell activation. J Immunol. 1990 Sep 1;145(5):1337–1342. [PubMed] [Google Scholar]
- Ray A., LaForge K. S., Sehgal P. B. On the mechanism for efficient repression of the interleukin-6 promoter by glucocorticoids: enhancer, TATA box, and RNA start site (Inr motif) occlusion. Mol Cell Biol. 1990 Nov;10(11):5736–5746. doi: 10.1128/mcb.10.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Sassone-Corsi P., Sehgal P. B. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol. 1989 Dec;9(12):5537–5547. doi: 10.1128/mcb.9.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Guyre P. M., Ball E. D., Fanger M. W. Glucocorticoid enhances gamma interferon effects on human monocyte antigen expression and ADCC. Clin Exp Immunol. 1986 Aug;65(2):387–395. [PMC free article] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ward P. A., Spengler R. N., Lynch J. P., 3rd, Larrick J., Kunkel S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Singhel K. J., Overholtzer J. F., Shurtleff S. A. Regulation of tumor necrosis factor expression in a macrophage-like cell line by lipopolysaccharide and cyclic AMP. Cell Immunol. 1989 May;120(2):291–300. doi: 10.1016/0008-8749(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Waage A., Slupphaug G., Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990 Nov;20(11):2439–2443. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- Waage A., Sørensen M., Størdal B. Differential effect of oxpentifylline on tumour necrosis factor and interleukin-6 production. Lancet. 1990 Mar 3;335(8688):543–543. doi: 10.1016/0140-6736(90)90779-5. [DOI] [PubMed] [Google Scholar]
- Zabel P., Wolter D. T., Schönharting M. M., Schade U. F. Oxpentifylline in endotoxaemia. Lancet. 1989 Dec 23;2(8678-8679):1474–1477. doi: 10.1016/s0140-6736(89)92929-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Yip Y. K., Vilcek J. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6802–6805. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lin J. X., Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988 May 5;263(13):6177–6182. [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R. A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991 Apr 1;146(7):2088–2094. [PubMed] [Google Scholar]



