Abstract
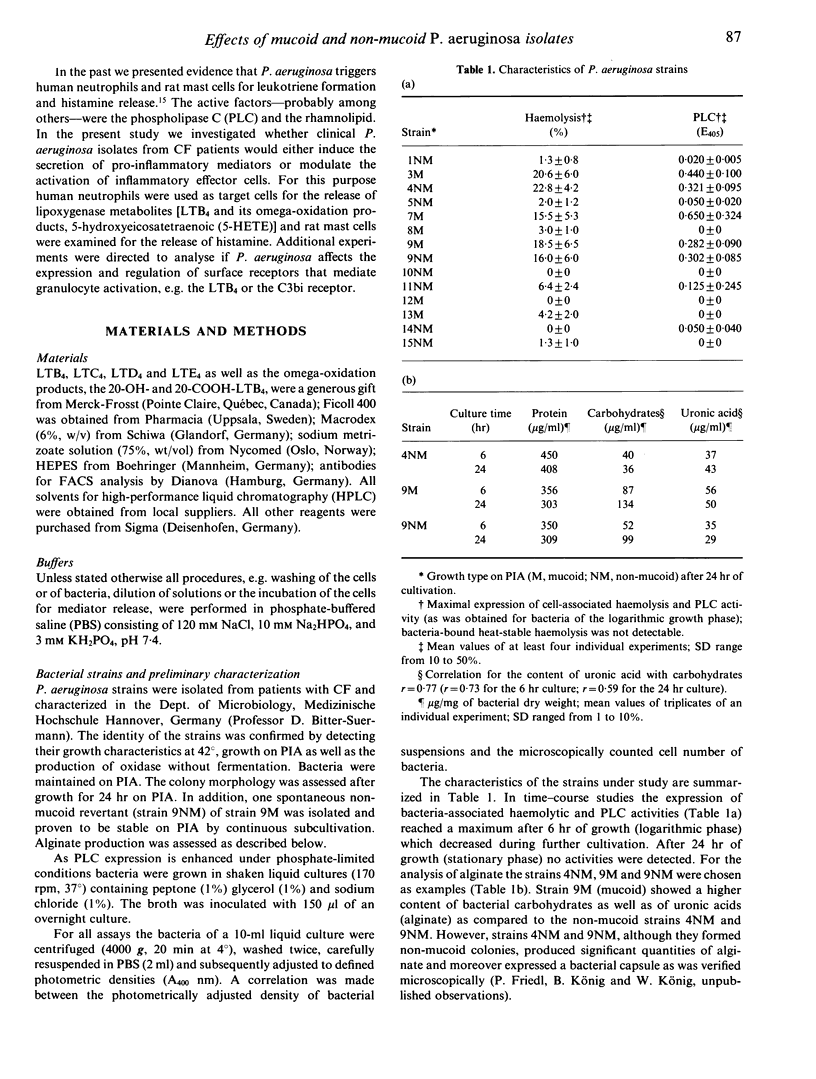
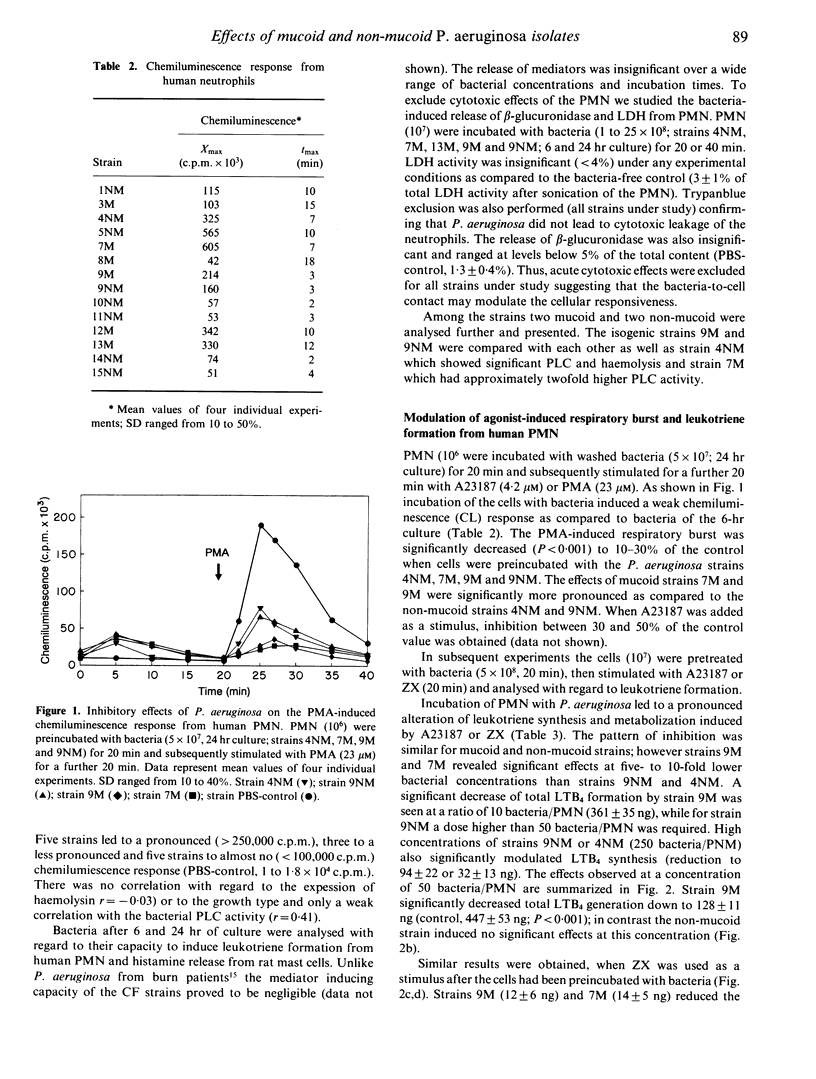
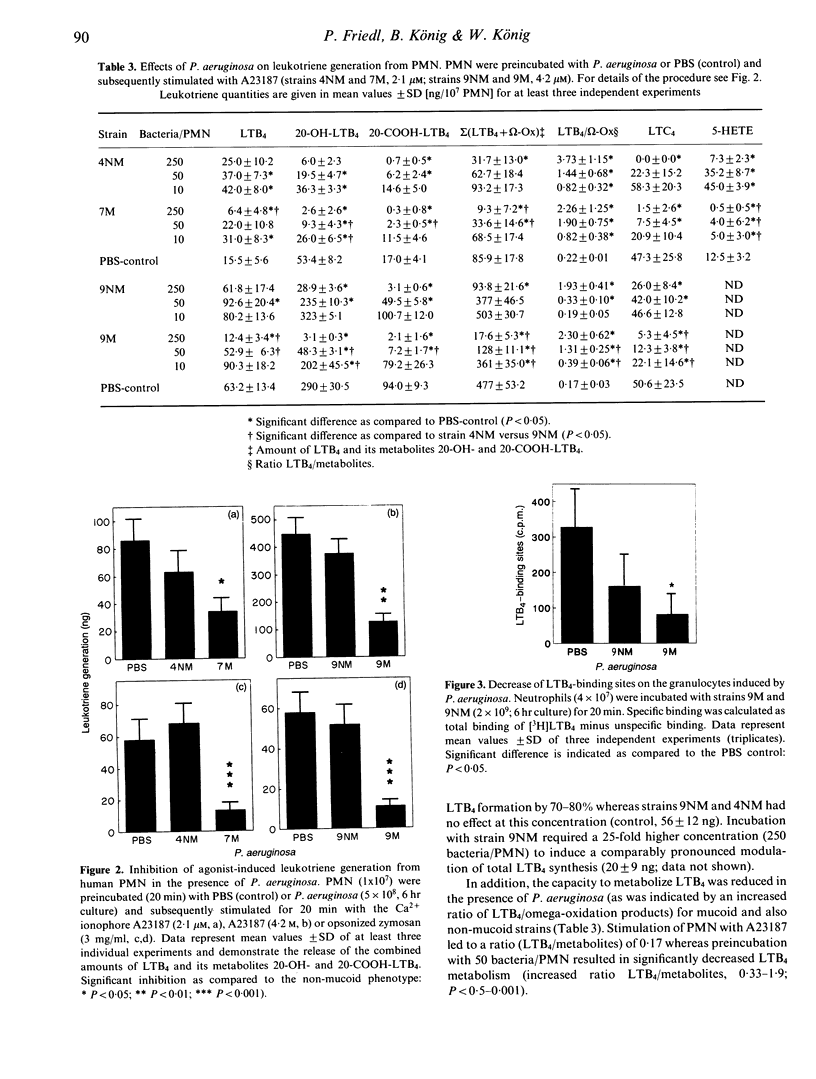
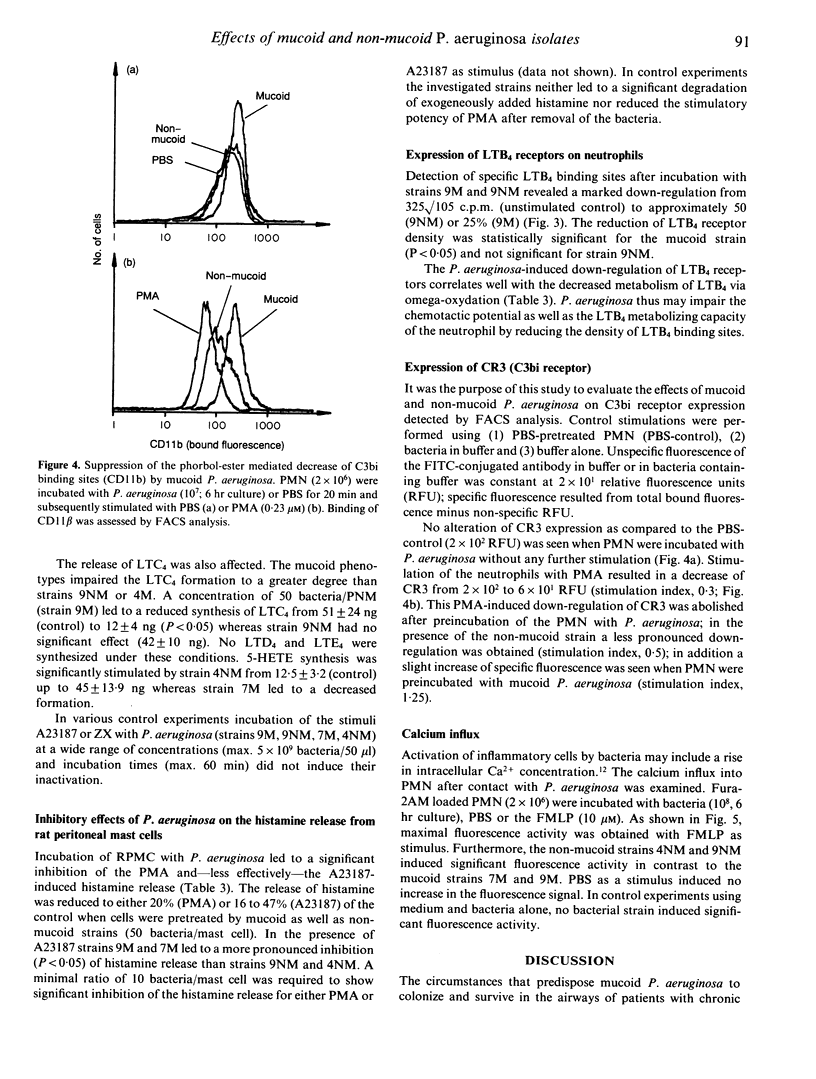
Mucoid Pseudomonas aeruginosa causing chronic bronchopulmonary infection in cystic fibrosis (CF) patients may interfere with host defence mechanisms. We investigated 13 P. aeruginosa strains isolated from sputa of CF patients with regard to the induction or modulation of inflammatory mediator release from human neutrophils (PMN) and rat mast cells. The effects of mucoid as compared to non-mucoid bacteria were studied using a mucoid strain and its non-mucoid revertant. The release of leukotrienes (LT) and histamine in response to the majority of the CF strains was insignificant. However, preincubation of PMN with P. aeruginosa caused a dose-dependent decrease (50-95%) of LTB4 and LTC4 generation and LTB4 metabolism induced by the Ca(2+)-ionophore A23187 or opsonized zymosan (ZX) (P less than 0.001). The mucoid strains caused a three- to 10-fold higher impairment of LTB4 release (P less than 0.05) and a concomitant down-regulation of LTB4 receptors on neutrophils. Inhibitory effects were also obtained for mucoid and non-mucoid bacteria when the phorbol-ester or the Ca(2+)-ionophore induced luminol enhanced chemiluminescence response (P less than 0.001) or the histamine release from rat peritoneal mast cells (P less than 0.01) was studied. The bacteria-cell contact with non-mucoid strains was associated with an increased Ca2+ influx into PMN, whereas mucoid bacteria had no effect. In addition, a protein kinase C-dependent decrease of the C3bi receptor was suppressed by the mucoid--and less effectively--by the non-mucoid strain. The results suggest that the impairment of the phagocytic and inflammatory system may contribute to the pathogenesis and persistence of mucoid P. aeruginosa infection in CF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann U., Scheffer J., Köller M., Schönfeld W., Erbs G., Müller F. E., König W. Induction of inflammatory mediators (histamine and leukotrienes) from rat peritoneal mast cells and human granulocytes by Pseudomonas aeruginosa strains from burn patients. Infect Immun. 1989 Jul;57(7):2187–2195. doi: 10.1128/iai.57.7.2187-2195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., Schönfeld W., König W. Metabolism of leukotriene B4 by activated human polymorphonuclear granulocytes. Immunology. 1988 Jul;64(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M., Matthews L. W. Polyuronic acids produced by Pseudomonas aeruginosa. Biochemistry. 1966 Sep;5(9):2817–2822. doi: 10.1021/bi00873a006. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. The enhancement of inflammatory injury. Am Rev Respir Dis. 1987 Jul;136(1):1–2. doi: 10.1164/ajrccm/136.1.1. [DOI] [PubMed] [Google Scholar]
- Doerfler M. E., Danner R. L., Shelhamer J. H., Parrillo J. E. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J Clin Invest. 1989 Mar;83(3):970–977. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Høiby N. Longitudinal study of immune response to Pseudomonas aeruginosa antigens in cystic fibrosis. Infect Immun. 1983 Oct;42(1):197–201. doi: 10.1128/iai.42.1.197-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar F., Speert D. P. Alginase treatment of mucoid Pseudomonas aeruginosa enhances phagocytosis by human monocyte-derived macrophages. Infect Immun. 1988 Nov;56(11):2788–2793. doi: 10.1128/iai.56.11.2788-2793.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett D. J., Cohen M. S. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989 Dec;3(14):2574–2582. doi: 10.1096/fasebj.3.14.2556311. [DOI] [PubMed] [Google Scholar]
- Høiby N. Cystic fibrosis: infection. Schweiz Med Wochenschr. 1991 Jan 26;121(4):105–109. [PubMed] [Google Scholar]
- Knöller J., Schönfeld W., Köller M., Hensler T., König W. Arachidonic acid metabolites from polymorphonuclear leukocytes of healthy donors, severely burned patients and children with cystic fibrosis--routine monitoring by high-performance liquid chromatography. J Chromatogr. 1988 Jun 3;427(2):199–208. doi: 10.1016/0378-4347(88)80122-1. [DOI] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Phosphorylcholine stimulates capsule formation of phosphate-limited mucoid Pseudomonas aeruginosa. Infect Immun. 1988 Apr;56(4):864–873. doi: 10.1128/iai.56.4.864-873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Helmke R. J., German V. F., Mangos J. A. Resistance of mucoid Pseudomonas aeruginosa to nonopsonic phagocytosis by alveolar macrophages in vitro. Infect Immun. 1988 Dec;56(12):3173–3179. doi: 10.1128/iai.56.12.3173-3179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., Schönfeld W., Raulf M., Köller M., Knöller J., Scheffer J., Brom J. The neutrophil and leukotrienes--role in health and disease. Eicosanoids. 1990;3(1):1–22. [PubMed] [Google Scholar]
- Laharrague P. F., Corberand J. X., Fillola G., Gleizes B. J., Fontanilles A. M., Gyrard E. In vitro effect of the slime of Pseudomonas aeruginosa on the function of human polymorphonuclear neutrophils. Infect Immun. 1984 Jun;44(3):760–762. doi: 10.1128/iai.44.3.760-762.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Jones R. S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J Biol Chem. 1966 Aug 25;241(16):3845–3851. [PubMed] [Google Scholar]
- Marcus H., Austria A., Baker N. R. Adherence of Pseudomonas aeruginosa to tracheal epithelium. Infect Immun. 1989 Apr;57(4):1050–1053. doi: 10.1128/iai.57.4.1050-1053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers D. J., Berk R. S. Characterization of phospholipase C from Pseudomonas aeruginosa as a potent inflammatory agent. Infect Immun. 1990 Mar;58(3):659–666. doi: 10.1128/iai.58.3.659-666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Tanaka S. Computer-aided calculation of amino acid composition of proteins. Anal Biochem. 1968 Aug;24(2):270–280. doi: 10.1016/0003-2697(68)90180-2. [DOI] [PubMed] [Google Scholar]
- Papini E., Grzeskowiak M., Bellavite P., Rossi F. Protein kinase C phosphorylates a component of NADPH oxidase of neutrophils. FEBS Lett. 1985 Oct 14;190(2):204–208. doi: 10.1016/0014-5793(85)81284-9. [DOI] [PubMed] [Google Scholar]
- Pedersen S. S., Espersen F., Høiby N., Jensen T. Immunoglobulin A and immunoglobulin G antibody responses to alginates from Pseudomonas aeruginosa in patients with cystic fibrosis. J Clin Microbiol. 1990 Apr;28(4):747–755. doi: 10.1128/jcm.28.4.747-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986 Aug;24(2):189–196. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Nicotra M. B. Pseudomonas aeruginosa mucoid strain. Its significance in adult chest diseases. Am Rev Respir Dis. 1982 Nov;126(5):833–836. doi: 10.1164/arrd.1982.126.5.833. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M. Leukotrienes and the immune system. J Lipid Mediat. 1989 May-Jun;1(3):149–159. [PubMed] [Google Scholar]
- Sampson A. P., Spencer D. A., Green C. P., Piper P. J., Price J. F. Leukotrienes in the sputum and urine of cystic fibrosis children. Br J Clin Pharmacol. 1990 Dec;30(6):861–869. doi: 10.1111/j.1365-2125.1990.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Braun V., Goebel W. Comparison of four hemolysin-producing organisms (Escherichia coli, Serratia marcescens, Aeromonas hydrophila, and Listeria monocytogenes) for release of inflammatory mediators from various cells. J Clin Microbiol. 1988 Mar;26(3):544–551. doi: 10.1128/jcm.26.3.544-551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Zakem H., Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990 Jul;86(1):300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler W. B., Williams M., Matthews W. J., Jr, Colten H. R. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: a 5-year longitudinal study. J Pediatr. 1984 May;104(5):695–699. doi: 10.1016/s0022-3476(84)80946-4. [DOI] [PubMed] [Google Scholar]
- Zakrzewski J. T., Barnes N. C., Costello J. F., Piper P. J. Lipid mediators in cystic fibrosis and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987 Sep;136(3):779–782. doi: 10.1164/ajrccm/136.3.779. [DOI] [PubMed] [Google Scholar]


