Abstract
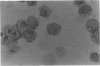
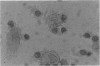
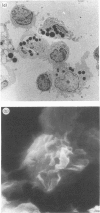
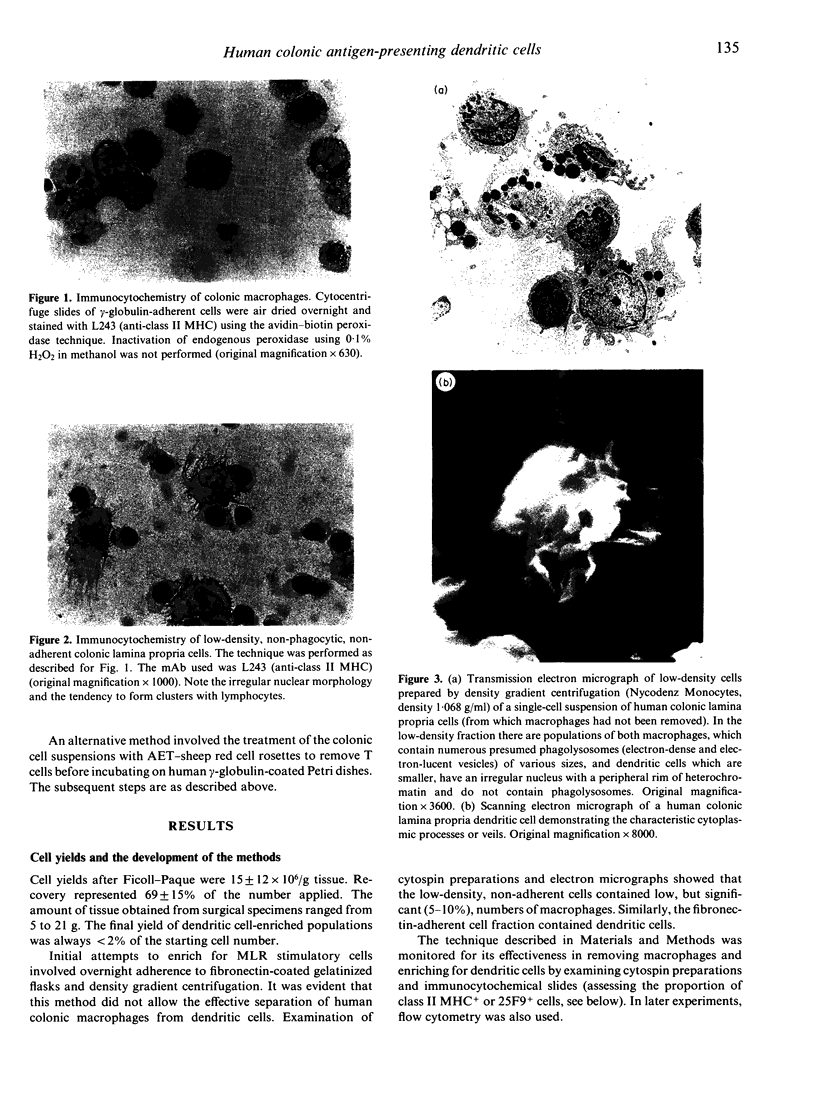
Induction of T-cell responses requires the recognition of antigen in association with class II major histocompatibility complex (MHC) antigens on specialized antigen-presenting cells. It was previously demonstrated that dendritic cells were the major antigen-presenting cell in the mouse intestinal lamina propria whilst macrophages were shown to be suppressive. The aim of this study was to compare the antigen-presenting cell activity of human colonic dendritic cells with macrophages. Colonic mucosa was removed from 46 specimens resected for cancer and other non-malignant conditions and lamina propria cell suspensions obtained by EDTA treatment followed by enzymatic digestion. Lamina propria cell suspensions, depleted of macrophages by adherence to insolubilized human immunoglobulin and carbonyl iron phagocytosis, were enriched for dendritic cells by density gradient centrifugation. Yields represented 0.9% (range 0.7-1.4%) of the starting cell number and the degree of enrichment was 30-50%. Immunocytochemistry demonstrated high levels of class II MHC antigen expression, but low levels or absent expression of macrophage and other markers. The ultrastructural features of the low-density cell fraction were typical of dendritic cells with cytoplasmic extensions or veils and the absence of phagocytic vesicles. Populations of cells enriched for macrophages were obtained by harvesting the human immunoglobulin-adherent cells. These cells were > 70% positive for macrophage markers using immunocytochemistry. The ability of lamina propria cells to induce primary T-cell activation was assayed using allogeneic peripheral blood T cells as responders in the mixed leucocyte reaction (MLR). When antigen-presenting activity was assessed using the MLR, the stimulatory activity was present in the dendritic cell-enriched fraction, with little activity present in the macrophage fraction. These data indicate that dendritic cells, not macrophages, are the major cell population capable of generating a mixed leucocyte reaction in the human colonic lamina propria.
Full text
PDF


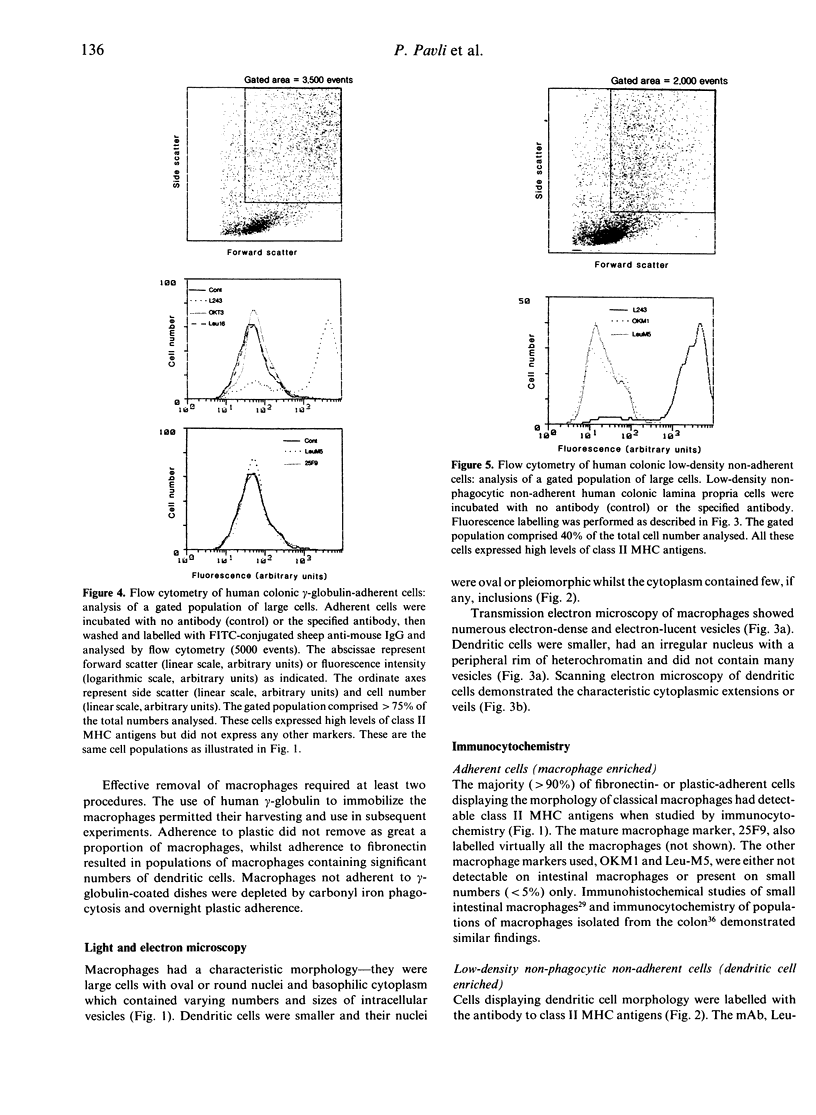
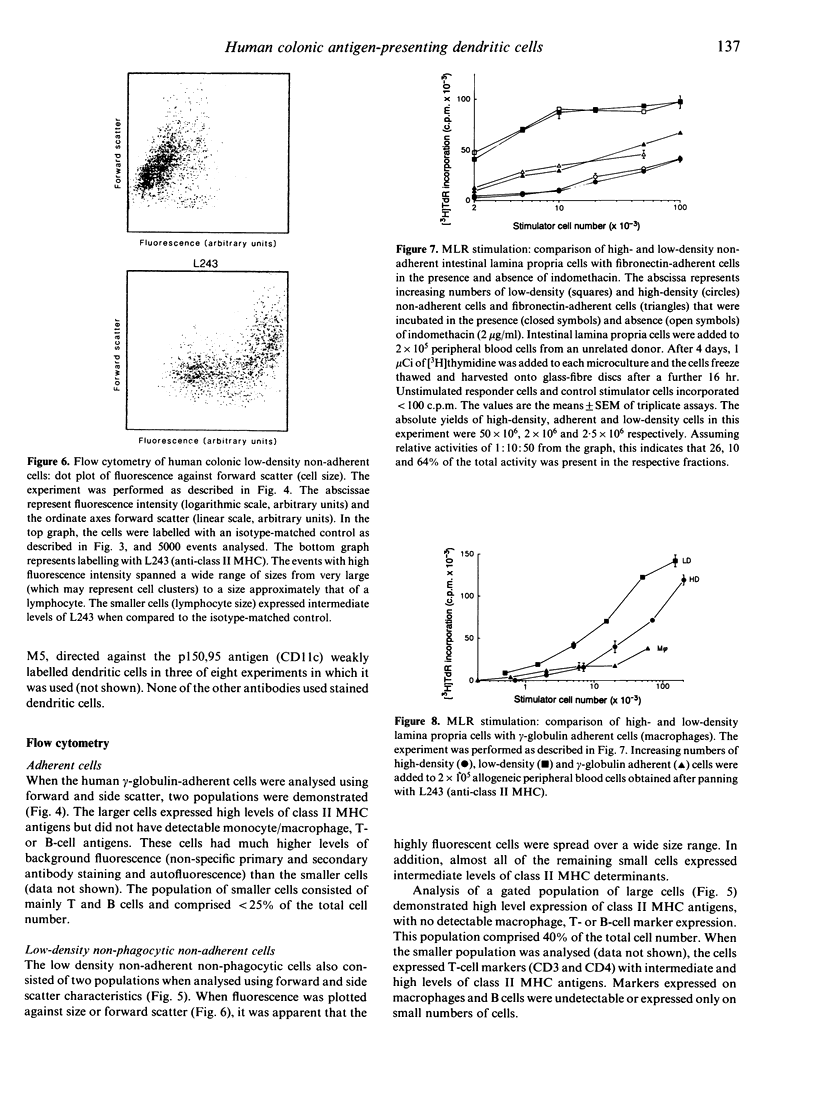




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Fabian R. J., Golberg L., Coulston F. Role of lysosomes in carrageenan-induced cecal ulceration. Gastroenterology. 1974 Dec;67(6):1169–1181. [PubMed] [Google Scholar]
- Austyn J. M. Lymphoid dendritic cells. Immunology. 1987 Oct;62(2):161–170. [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Bookman M. A., Bull D. M. Characteristics of isolated intestinal mucosal lymphoid cells in inflammatory bowel disease. Gastroenterology. 1979 Sep;77(3):503–510. [PubMed] [Google Scholar]
- Bujdoso R., Hopkins J., Dutia B. M., Young P., McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989 Oct 1;170(4):1285–1301. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol. 1985;5(3):263–316. [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M., Inaba K., Steinman R. M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J Exp Med. 1990 Jul 1;172(1):383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fireman E. M., Ben Efraim S., Greif J., Kivity S., Topilsky M. R. Suppressor cell activity of human alveolar macrophages in interstitial lung diseases. Clin Exp Immunol. 1988 Jul;73(1):111–116. [PMC free article] [PubMed] [Google Scholar]
- Fireman E., Ben Efraim S., Greif J., Alguetti A., Ayalon D., Topilsky M. Correlation between PGE2 production and suppressor activity of alveolar macrophages from patients with interstitial lung diseases. Immunol Lett. 1988 Jun;18(2):159–165. doi: 10.1016/0165-2478(88)90058-2. [DOI] [PubMed] [Google Scholar]
- Flechner E. R., Freudenthal P. S., Kaplan G., Steinman R. M. Antigen-specific T lymphocytes efficiently cluster with dendritic cells in the human primary mixed-leukocyte reaction. Cell Immunol. 1988 Jan;111(1):183–195. doi: 10.1016/0008-8749(88)90062-7. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Gaudernack G., Bjercke S. Dendritic cells and monocytes as accessory cells in T-cell responses in man. I. Phenotypic analysis of dendritic cells and monocytes. Scand J Immunol. 1985 May;21(5):493–500. doi: 10.1111/j.1365-3083.1985.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988 Jul 1;168(1):157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Allan W., Hogan P. G., Doe W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987 Nov;42(5):474–484. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- Hume D. A. Immunohistochemical analysis of murine mononuclear phagocytes that express class II major histocompatibility antigens. Immunobiology. 1985 Dec;170(5):381–389. doi: 10.1016/S0171-2985(85)80062-0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Perry V. H., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec. 1984 Nov;210(3):503–512. doi: 10.1002/ar.1092100311. [DOI] [PubMed] [Google Scholar]
- Inaba K., Metlay J. P., Crowley M. T., Steinman R. M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990 Aug 1;172(2):631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Poulter L. W., Rawlings E., Burford G. D., Navarrete C., Ziegler A., Kelemen E. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol. 1986 Jun 15;136(12):4354–4361. [PubMed] [Google Scholar]
- Kabelitz D., Enssle K. H., Fleischer B., Reimann J. Antigen-presenting T cells. II. Clonal responses of alloreactive and virus-specific self-restricted human cytotoxic T cell responses stimulated by T lymphoblasts. J Immunol. 1987 Jan 1;138(1):45–50. [PubMed] [Google Scholar]
- Kaye J., Hedrick S. M. Analysis of specificity for antigen, Mls, and allogenic MHC by transfer of T-cell receptor alpha- and beta-chain genes. Nature. 1988 Dec 8;336(6199):580–583. doi: 10.1038/336580a0. [DOI] [PubMed] [Google Scholar]
- Knight S. C. Veiled cells--"dendritic cells" of the peripheral lymph. Immunobiology. 1984 Dec;168(3-5):349–361. doi: 10.1016/S0171-2985(84)80122-9. [DOI] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- MacPherson G. G. Properties of lymph-borne (veiled) dendritic cells in culture. I. Modulation of phenotype, survival and function: partial dependence on GM-CSF. Immunology. 1989 Sep;68(1):102–107. [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G., Pugh C. W. Heterogeneity amongst lymph-borne "dendritic" cells. Immunobiology. 1984 Dec;168(3-5):338–348. doi: 10.1016/S0171-2985(84)80121-7. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Characterization of antigen-presenting activity of intestinal mononuclear cells isolated from normal and inflammatory bowel disease colon and ileum. Immunology. 1988 Dec;65(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Pugh C. W., Webb M. The rat mixed lymphocyte reaction: roles of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981 Sep;44(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Holt P. G., Papadimitriou J. M. Functional characteristics of the veiled cells in afferent lymph from the rat intestine. Immunology. 1986 Jul;58(3):379–387. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Prickett T. C., Hart D. N. Human dendritic cells stimulate allogeneic T cells in the absence of IL-1. Immunology. 1989 Jul;67(3):290–297. [PMC free article] [PubMed] [Google Scholar]
- Monick M., Glazier J., Hunninghake G. W. Human alveolar macrophages suppress interleukin-1 (IL-1) activity via the secretion of prostaglandin E2. Am Rev Respir Dis. 1987 Jan;135(1):72–77. doi: 10.1164/arrd.1987.135.1.72. [DOI] [PubMed] [Google Scholar]
- Nunez G., Ball E. J., Stastny P. Accessory cell function of human endothelial cells. I. A subpopulation of Ia positive cells is required for antigen presentation. J Immunol. 1983 Aug;131(2):666–673. [PubMed] [Google Scholar]
- Pavli P., Woodhams C. E., Doe W. F., Hume D. A. Isolation and characterization of antigen-presenting dendritic cells from the mouse intestinal lamina propria. Immunology. 1990 May;70(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M. Isolation of large mononuclear Ia-positive veiled cells from the mouse thoracic duct. J Immunol Methods. 1985 Dec 27;85(2):383–392. doi: 10.1016/0022-1759(85)90147-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Escamilla E., Torres N. Experimental Campylobacter diarrhea in chickens. Infect Immun. 1981 Oct;34(1):250–255. doi: 10.1128/iai.34.1.250-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Spalding D. M., Koopman W. J., Eldridge J. H., McGhee J. R., Steinman R. M. Accessory cells in murine Peyer's patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983 May 1;157(5):1646–1659. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., MacDonald T. T., Isaacson P. G. Heterogeneity of non-lymphoid cells expressing HLA-D region antigens in human fetal gut. Clin Exp Immunol. 1987 Feb;67(2):415–424. [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Inaba K. Stimulation of the primary mixed leukocyte reaction. Crit Rev Immunol. 1985;5(4):331–348. [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Thepen T., Van Rooijen N., Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989 Aug 1;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. A method for the production and quantitative assay of human lymphokine preparations. J Immunol Methods. 1981;41(1):9–21. doi: 10.1016/0022-1759(81)90269-6. [DOI] [PubMed] [Google Scholar]
- Warren H. S. The generation of specific cytotoxic responses by human lymphocytes requires antigen and activities provided by a lymphokine supernatant. Scand J Immunol. 1981 Jul;14(1):71–76. doi: 10.1111/j.1365-3083.1981.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Wilders M. M., Drexhage H. A., Kokjé M., Verspaget H. W., Meuwissen S. G. Peripolesis followed by cytotoxicity in chronic idiopathic inflammatory bowel disease. Clin Exp Immunol. 1984 Sep;57(3):614–620. [PMC free article] [PubMed] [Google Scholar]
- Wilders M. M., Drexhage H. A., Kokjé M., Verspaget H. W., Meuwissen S. G. Veiled cells in chronic idiopathic inflammatory bowel disease. Clin Exp Immunol. 1984 Feb;55(2):377–387. [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Zwadlo G., Bröcker E. B., von Bassewitz D. B., Feige U., Sorg C. A monoclonal antibody to a differentiation antigen present on mature human macrophages and absent from monocytes. J Immunol. 1985 Mar;134(3):1487–1492. [PubMed] [Google Scholar]