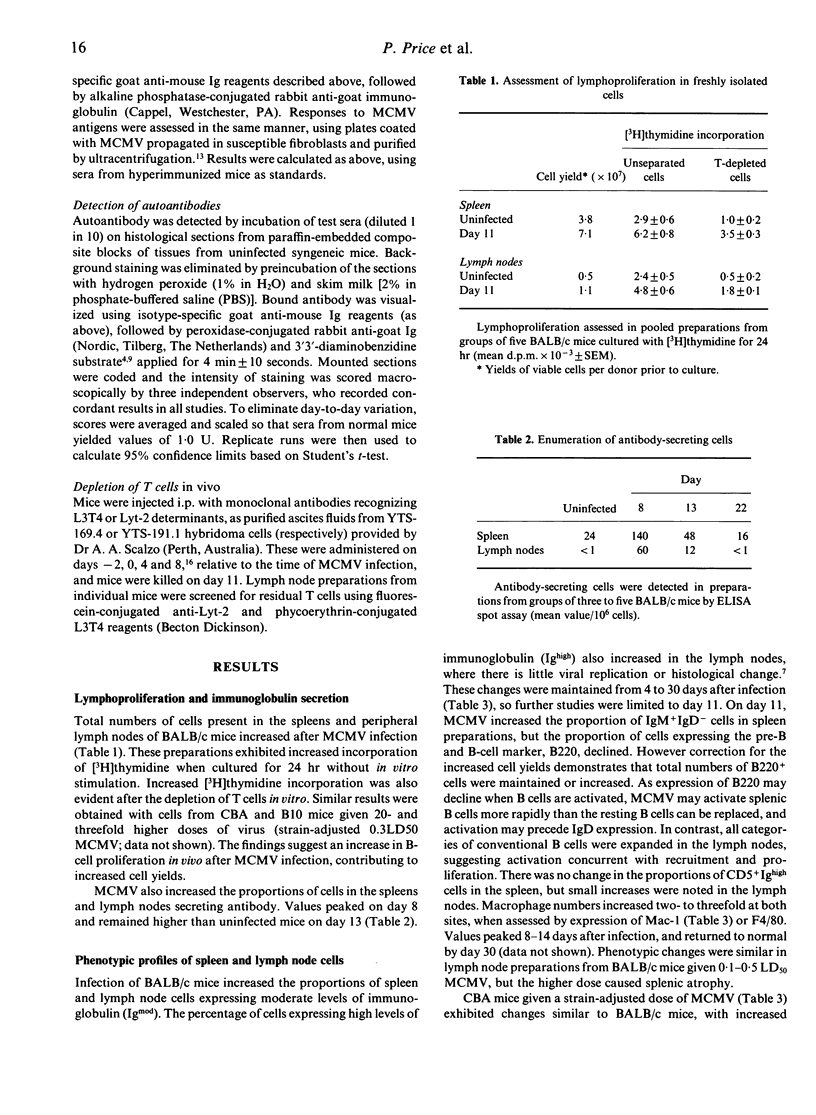
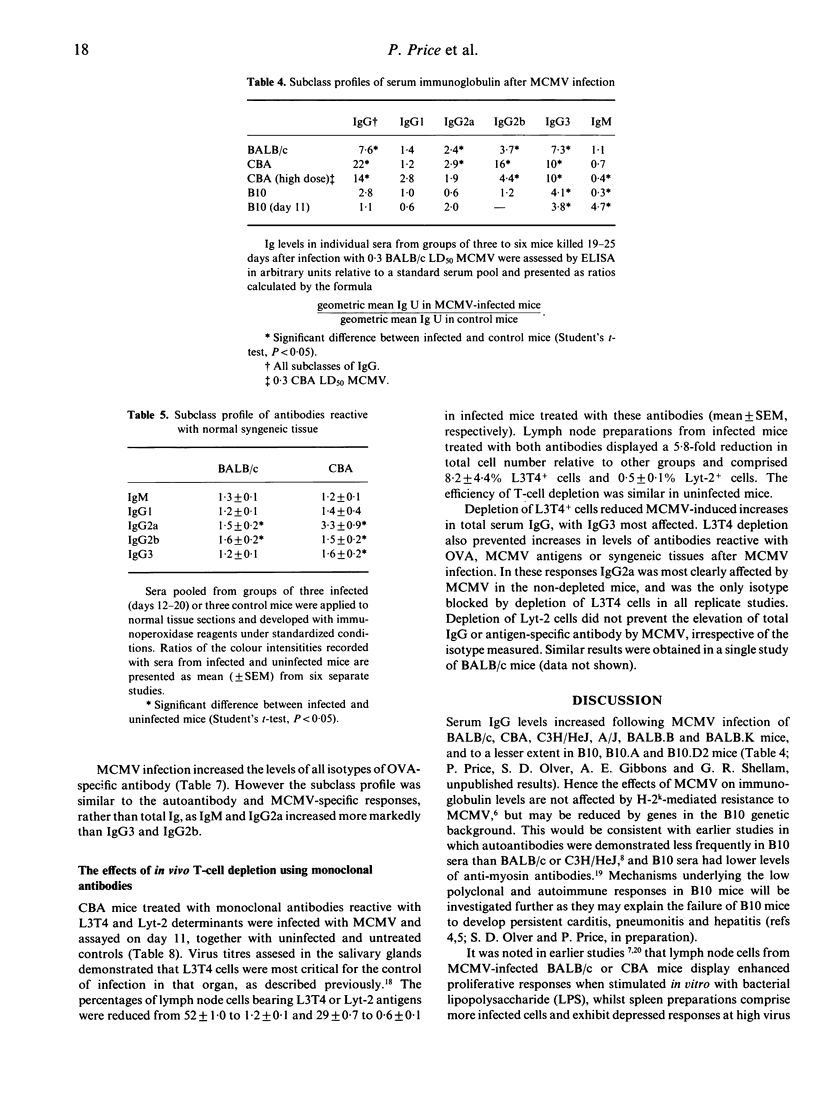
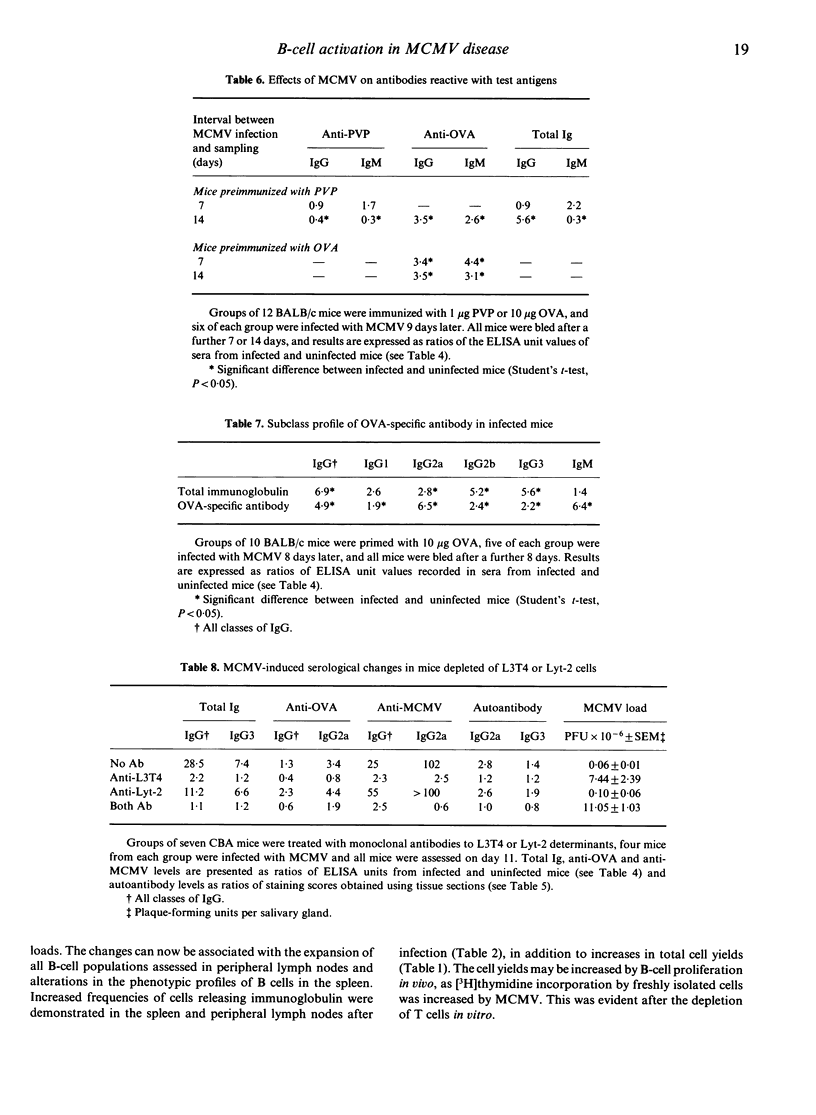
Abstract
Infection of susceptible mice with murine cytomegalovirus (MCMV) induces persistent inflammation, and the production of autoantibodies reactive with large numbers of proteins from all major organs. However the roles of polyclonal B-cell activation, autoreactive T-helper cells and host-virus cross-reactions in these phenomena have not been evaluated. The present study reveals six- to 20-fold increases in serum immunoglobulin levels in MCMV-infected BALB/c and CBA mice, with IgG3 and IgG2b most affected. Titres of antibodies reactive with autologous tissues and ovalbumin (OVA) also increased following MCMV infection, whilst responses to a synthetic antigen [polyvinyl pyrrolidone (PVP)] were unaffected or depressed. IgG2a was the isotype most affected in responses to OVA, MCMV antigens and autologous tissues, suggesting interferon-gamma (IFN-gamma) may contribute to responses induced in the presence of the relevant antigen. Increases in total and antigen-specific immunoglobulin levels were CD4 dependent, as they were reduced in infected mice depleted of these cells with anti-CD4 antibodies. Serological changes were preceded by B-cell expansion and activation evident from increased cell yields, frequencies of cells releasing immunoglobulin and proliferation of T-depleted spleen and lymph node preparations. Numbers of mature B cells and macrophages increased in the lymph nodes, but B-1a (CD5+ Ig+) cell counts remained low. Alterations in the B-cell phenotypic profiles were more complex in the spleen, but correction for increased cell yields revealed increases in some subpopulations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Boldogh I., Fons M., Lee C. H., AbuBakar S., Russell J. M., Au W. W. Cell-activation responses to cytomegalovirus infection relationship to the phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. [PubMed] [Google Scholar]
- Amadori A., Chieco-Bianchi L. B-cell activation and HIV-1 infection: deeds and misdeeds. Immunol Today. 1990 Oct;11(10):374–379. doi: 10.1016/0167-5699(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Andersen H. K. Smooth-muscle antibodies and other tissue antibodies in cytomegalovirus infection. Clin Exp Immunol. 1975 Oct;22(1):22–29. [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., O'Donoghue H., Foti D., Lawson C. M., Shellam G. R., Reed W. D. Multiple autoantibodies following cytomegalovirus infection: virus distribution and specificity of autoantibodies. Immunology. 1988 Jul;64(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Becker H., Weber C., Storch S., Federlin K. Relationship between CD5+ B lymphocytes and the activity of systemic autoimmunity. Clin Immunol Immunopathol. 1990 Aug;56(2):219–225. doi: 10.1016/0090-1229(90)90143-e. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., Van Snick J. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol. 1985 Mar;15(3):250–255. doi: 10.1002/eji.1830150308. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Characterization of neutralizing monoclonal antibodies to murine cytomegalovirus. J Gen Virol. 1990 Mar;71(Pt 3):655–664. doi: 10.1099/0022-1317-71-3-655. [DOI] [PubMed] [Google Scholar]
- Griffiths P. D., Grundy J. E. The status of CMV as a human pathogen. Epidemiol Infect. 1988 Feb;100(1):1–15. doi: 10.1017/s095026880006550x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Walker D. G., Altamirano M. Analysis in vitro of two biologically distinct strains of murine cytomegalovirus. Arch Virol. 1988;102(3-4):289–295. doi: 10.1007/BF01310834. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Koszinowski U. H. Molecular aspects of immune recognition of cytomegalovirus. Transplant Proc. 1991 Jun;23(3 Suppl 3):70-3, discussion 74. [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988 Aug;69(Pt 8):1987–1998. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Farrell H. E., Shellam G. R., Reed W. D. Murine anti-cytomegalovirus monoclonal antibodies with autoreactivity. Immunology. 1991 Mar;72(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Adam E., DeBakey M. E. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990 Apr 25;263(16):2204–2207. [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- O'Donoghue H. L., Lawson C. M., Reed W. D. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology. 1990 Sep;71(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- Price P. Depression of humoral responses by murine cytomegalovirus infection. Immunol Cell Biol. 1990 Feb;68(Pt 1):33–43. doi: 10.1038/icb.1990.5. [DOI] [PubMed] [Google Scholar]
- Price P., Eddy K. S., Papadimitriou J. M., Faulkner D. L., Shellam G. R. Genetic determination of cytomegalovirus-induced and age-related cardiopathy in inbred mice. Characterization of infiltrating cells. Am J Pathol. 1991 Jan;138(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- Price P., Gibbons A. E., Shellam G. R. H-2 class I loci determine sensitivity to MCMV in macrophages and fibroblasts. Immunogenetics. 1990;32(1):20–26. doi: 10.1007/BF01787324. [DOI] [PubMed] [Google Scholar]
- Price P., Winter J. G., Eddy K. S., Shellam G. R. Inflammatory and immunological responses to murine cytomegalovirus in resistant CBA mice. Arch Virol. 1989;104(1-2):35–51. doi: 10.1007/BF01313806. [DOI] [PubMed] [Google Scholar]
- Price P., Winter J. G., Shellam G. R. The inflammatory macrophage response to murine cytomegalovirus in genetically susceptible mice. Arch Virol. 1989;106(1-2):35–50. doi: 10.1007/BF01311036. [DOI] [PubMed] [Google Scholar]
- Prowse S. J., Ey P. L., Jenkin C. R. Immunity to Nematospiroides dubius: cell and immunoglobulin changes associated with the onset of immunity in mice. Aust J Exp Biol Med Sci. 1978 Apr;56(2):237–246. doi: 10.1038/icb.1978.25. [DOI] [PubMed] [Google Scholar]
- Scalzo A. A., Fitzgerald N. A., Wallace C. R., Gibbons A. E., Smart Y. C., Burton R. C., Shellam G. R. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J Immunol. 1992 Jul 15;149(2):581–589. [PubMed] [Google Scholar]
- Schattner A., Rager-Zisman B. Virus-induced autoimmunity. Rev Infect Dis. 1990 Mar-Apr;12(2):204–222. doi: 10.1093/clinids/12.2.204. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Shanley J. D. Host genetic factors influence murine cytomegalovirus lung infection and interstitial pneumonitis. J Gen Virol. 1984 Dec;65(Pt 12):2121–2128. doi: 10.1099/0022-1317-65-12-2121. [DOI] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]



