Abstract
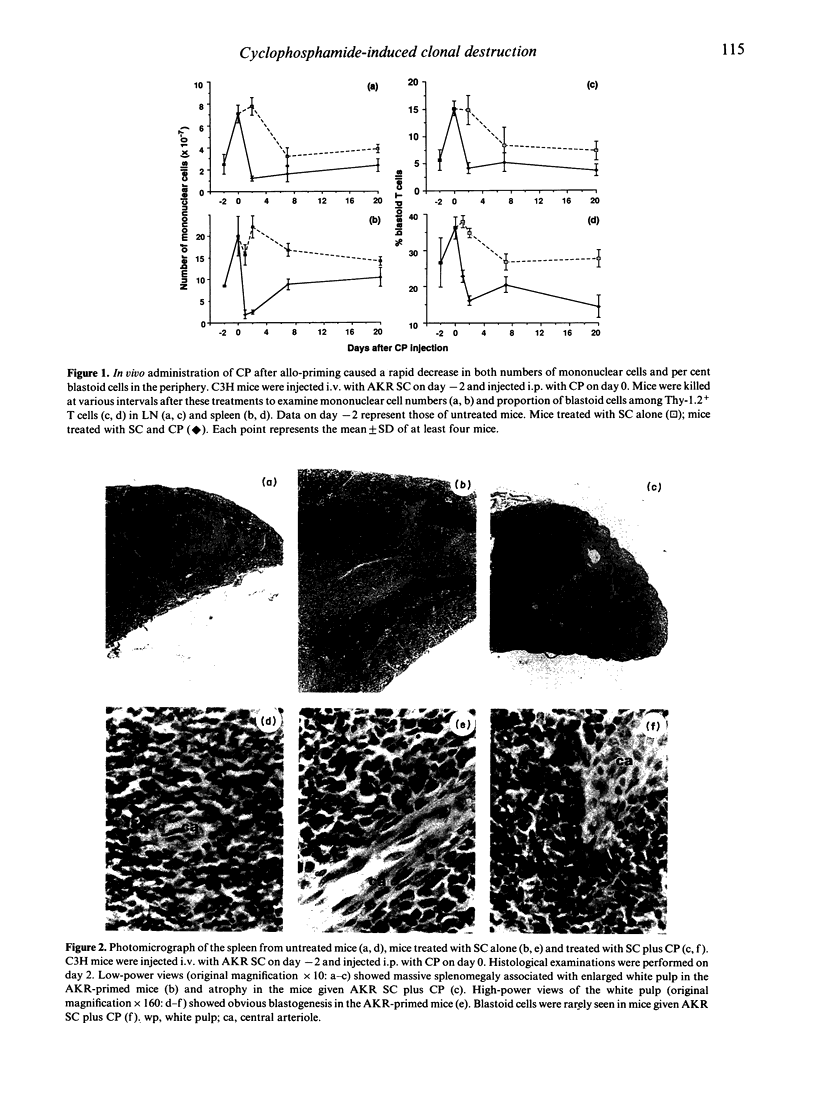
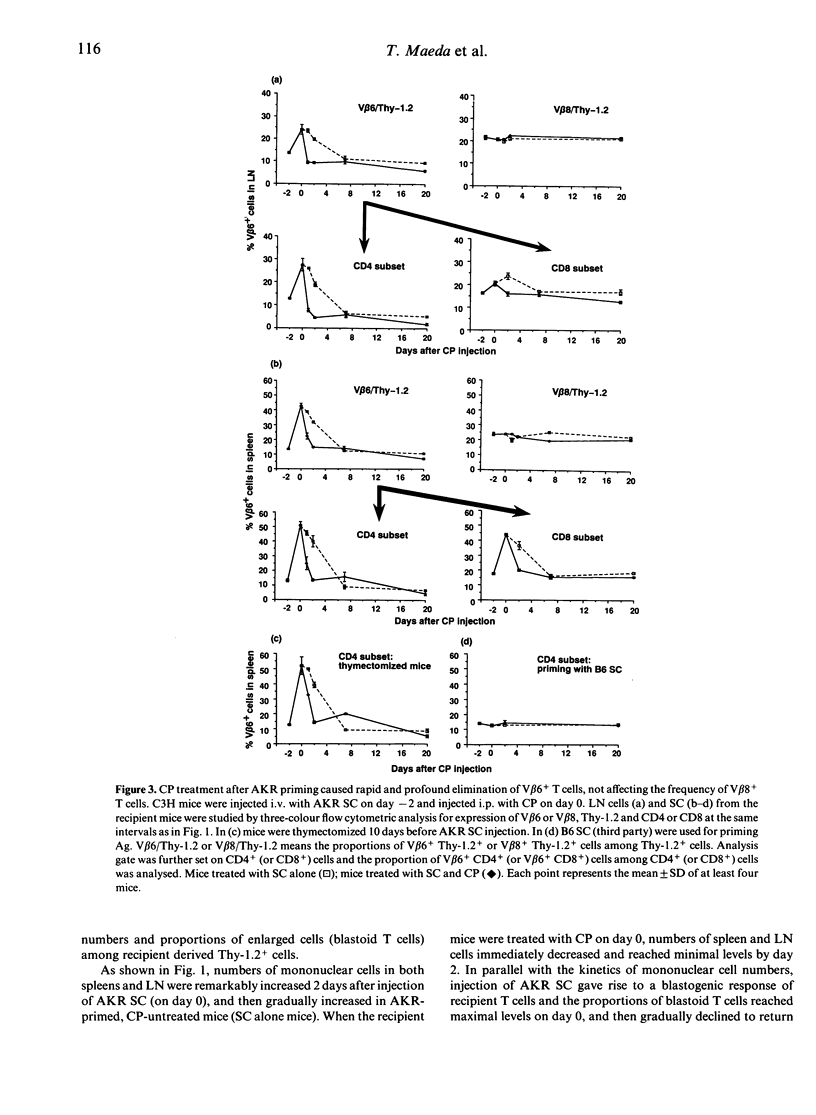
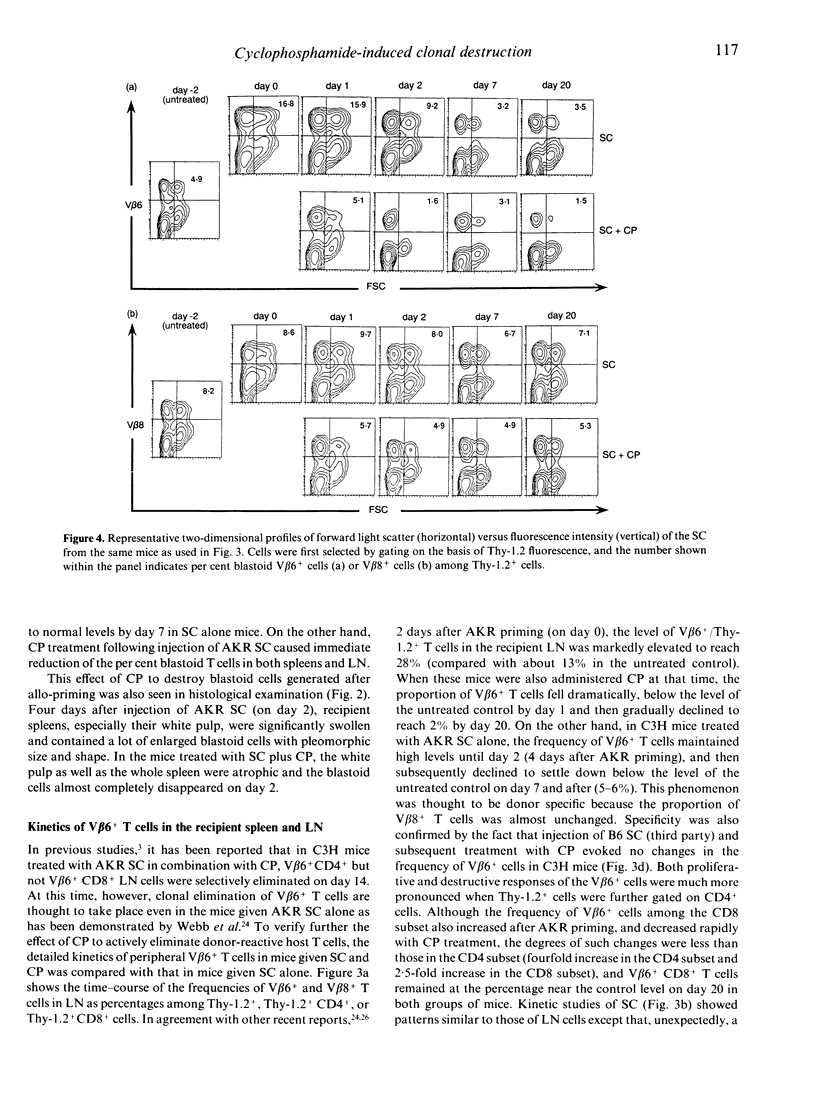
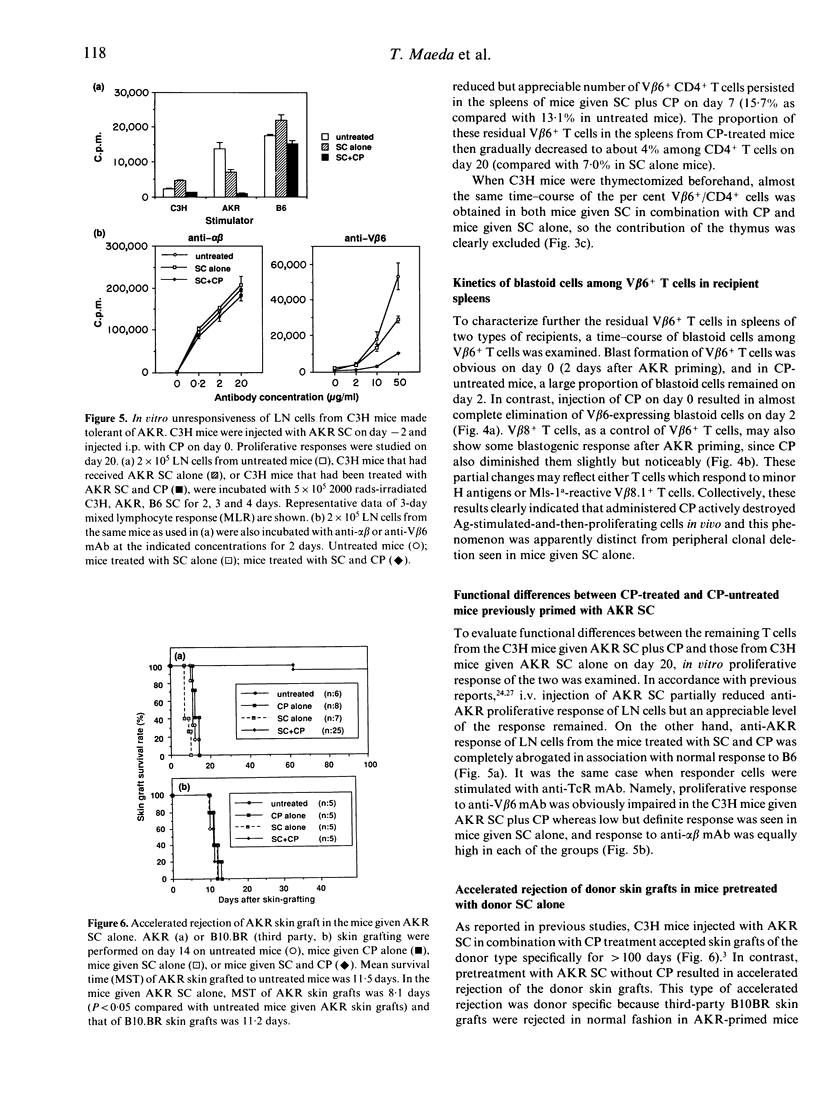
It has previously been reported that a single i.p. injection of 200 mg/kg cyclophosphamide (CP) 2 days after priming with 10(8) donor spleen cells (SC) leads to donor-specific skin allograft tolerance in H-2 compatible, multiminor antigen incompatible murine strain combinations. It is speculated that the i.v. injection of donor cells may result in synchronized proliferation of donor-reactive host T cells and subsequently administered CP may specifically destroy these proliferating T cells in the periphery. Although this unique action of CP is considered to be a principal mechanism in this method, direct evidence has not yet been obtained. In the present article, this in vivo destructive effect of CP is clearly demonstrated by assessing detailed kinetics of host-derived blastoid T cells and donor (Mls-1a)-reactive V beta 6+ T cells in the model system of C3H mice rendered tolerant to AKR. Frequencies of the blastoid cells and V beta 6+ cells, which increased as a result of AKR priming, decreased rapidly with the administration of CP. C3H mice, which received AKR SC alone, also exhibited partial deletion of V beta 6+ T cells, but both tempo and magnitude of decrease in the frequency of V beta 6+ cells were quite different from those of the C3H mice given AKR SC and CP, which showed more rapid and profound elimination of V beta 6+ T cells. In accordance with these kinetic studies, in vitro proliferative response to Mls-1a antigens was greatly impaired in mice treated with SC and CP, whereas a low but appreciable response was detected in mice given SC alone.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C. Studies on cyclophosphamide-induced tolerance to sheep erythrocytes. J Exp Med. 1967 May 1;125(5):833–845. doi: 10.1084/jem.125.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B. Immunological tolerance induced by cyclophosphamide assayed by plaque spleen cell method. Nature. 1967 Feb 4;213(5075):498–499. doi: 10.1038/213498a0. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bagley C. M., Jr, Bostick F. W., DeVita V. T., Jr Clinical pharmacology of cyclophosphamide. Cancer Res. 1973 Feb;33(2):226–233. [PubMed] [Google Scholar]
- Calne R. Y. Immunosuppression for organ grafting -- observations on cyclosporin A. Immunol Rev. 1979;46:113–124. doi: 10.1111/j.1600-065x.1979.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Chernyakhovskaya I. Y., Nagurskaya E. V., Shaposhnikova G. B., Prigozhina T. B., Fontalin L. N. Tolerance to allogeneic and to xenogeneic heart grafts provided by thymectomy of adult mice combined with donor cell and cyclophosphamide inoculation. Transplantation. 1980 May;29(5):409–412. doi: 10.1097/00007890-198005000-00013. [DOI] [PubMed] [Google Scholar]
- Dannecker G., Mecheri S., Staiano-Coico L., Hoffmann M. K. A characteristic Mls-1a response precedes Mls-1a anergy in vivo. J Immunol. 1991 Apr 1;146(7):2083–2087. [PubMed] [Google Scholar]
- Eto M., Mayumi H., Tomita Y., Yoshikai Y., Nishimura Y., Maeda T., Ando T., Nomoto K. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991 Mar 1;146(5):1402–1409. [PubMed] [Google Scholar]
- Eto M., Mayumi H., Tomita Y., Yoshikai Y., Nishimura Y., Nomoto K. Sequential mechanisms of cyclophosphamide-induced skin allograft tolerance including the intrathymic clonal deletion followed by late breakdown of the clonal deletion. J Immunol. 1990 Sep 1;145(5):1303–1310. [PubMed] [Google Scholar]
- Eto M., Mayumi H., Tomita Y., Yoshikai Y., Nishimura Y., Nomoto K. The requirement of intrathymic mixed chimerism and clonal deletion for a long-lasting skin allograft tolerance in cyclophosphamide-induced tolerance. Eur J Immunol. 1990 Sep;20(9):2005–2013. doi: 10.1002/eji.1830200919. [DOI] [PubMed] [Google Scholar]
- Eto M., Mayumi H., Tomita Y., Yoshikai Y., Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls-disparate antigens. J Exp Med. 1990 Jan 1;171(1):97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre J. W., Morris P. J. The effect of donor strain blood pretreatment on renal allograft rejection in rats. Transplantation. 1972 Nov;14(5):608–617. doi: 10.1097/00007890-197211000-00013. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Eckhaus M., Auchincloss H., Jr, Sachs D. H., Bluestone J. A. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [PubMed] [Google Scholar]
- Ildstad S. T., Sachs D. H. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984 Jan 12;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- Jardine I., Fenselau C., Appler M., Kan M. N., Brundrett R. B., Colvin M. Quantitation by gas chromatography-chemical ionization mass spectrometry of cyclophosphamide, phosphoramide mustard, and nornitrogen mustard in the plasma and urine of patients receiving cyclophosphamide therapy. Cancer Res. 1978 Feb;38(2):408–415. [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Longo D. L., Kruisbeek A. M. Peripheral clonal elimination of functional T cells. Science. 1990 Dec 21;250(4988):1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Baschieri S., Lees R. K. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991 Aug;21(8):1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Chvatchko Y. CD8+ T cells respond clonally to Mls-1a-encoded determinants. J Exp Med. 1990 Apr 1;171(4):1381–1386. doi: 10.1084/jem.171.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Pedrazzini T., Schneider R., Louis J. A., Zinkernagel R. M., Hengartner H. Intrathymic elimination of Mlsa-reactive (V beta 6+) cells during neonatal tolerance induction to Mlsa-encoded antigens. J Exp Med. 1988 Jun 1;167(6):2005–2010. doi: 10.1084/jem.167.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi H., Good R. A. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989 Jan 1;169(1):213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi H., Himeno K., Shin T., Nomoto K. Drug-induced tolerance to allografts in mice. VI. Tolerance induction in H-2-haplotype-identical strain combinations in mice. Transplantation. 1985 Aug;40(2):188–194. doi: 10.1097/00007890-198508000-00016. [DOI] [PubMed] [Google Scholar]
- Mayumi H., Nomoto K., Good R. A. A surgical technique for experimental free skin grafting in mice. Jpn J Surg. 1988 Sep;18(5):548–557. doi: 10.1007/BF02471489. [DOI] [PubMed] [Google Scholar]
- Okazaki H., Maki T., Wood M., Monaco A. P. Effect of a single transfusion of donor-specific and nonspecific blood on skin allograft survival in mice. Transplantation. 1980 Dec;30(6):421–424. doi: 10.1097/00007890-198012000-00007. [DOI] [PubMed] [Google Scholar]
- Opelz G., Sengar D. P., Mickey M. R., Terasaki P. I. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973 Mar;5(1):253–259. [PubMed] [Google Scholar]
- Qin S. X., Cobbold S., Benjamin R., Waldmann H. Induction of classical transplantation tolerance in the adult. J Exp Med. 1989 Mar 1;169(3):779–794. doi: 10.1084/jem.169.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J., Watanabe K., Hirasawa H., Cannon F. D., Mollen N., Blumenstock D. A., Ayvazian J. H., Ferrebee J. W. Cellular factors. Immunologic tolerance: irradiation and bone marrow transplantation in induction of canine allogeneic unresponsiveness. Transplant Proc. 1977 Mar;9(1):891–894. [PubMed] [Google Scholar]
- Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990 Oct 1;172(4):1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos G. W., Owens A. H., Jr 19S and 17S antibody production in the cyclophosphamide- or methotrexate-treated rat. Nature. 1966 Feb 5;209(5023):622–624. doi: 10.1038/209622a0. [DOI] [PubMed] [Google Scholar]
- Sato S., Azuma T., Shimizu J., Shima J., Kitagawa S., Hamaoka T., Fujiwara H. Property of class I H-2 alloantigen-reactive Lyt-2+ helper T cell subset. Abrogation of its proliferative and IL-2-producing capacities by intravenous injection of class I H-2-disparate allogeneic cells. J Immunol. 1988 Aug 1;141(3):721–727. [PubMed] [Google Scholar]
- Shizuru J. A., Gregory A. K., Chao C. T., Fathman C. G. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987 Jul 17;237(4812):278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- Slavin S., Strober S., Fuks Z., Kaplan H. S. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977 Jul 1;146(1):34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Stockman G. D., Trentin J. J. Cyclophosphamide-induced tolerance to equine globulin and to equine-anti-mouse-thymocyte globulin in adult mice. I. Studies on antigen and drug requirements. J Immunol. 1972 Jan;108(1):112–118. [PubMed] [Google Scholar]
- Tomita Y., Mayumi H., Eto M., Nomoto K. Importance of suppressor T cells in cyclophosphamide-induced tolerance to the non-H-2-encoded alloantigens. Is mixed chimerism really required in maintaining a skin allograft tolerance? J Immunol. 1990 Jan 15;144(2):463–473. [PubMed] [Google Scholar]
- Tomita Y., Nishimura Y., Harada N., Eto M., Ayukawa K., Yoshikai Y., Nomoto K. Evidence for involvement of clonal anergy in MHC class I and class II disparate skin allograft tolerance after the termination of intrathymic clonal deletion. J Immunol. 1990 Dec 15;145(12):4026–4036. [PubMed] [Google Scholar]
- Webb S. R., Sprent J. Response of mature unprimed CD8+ T cells to Mlsa determinants. J Exp Med. 1990 Mar 1;171(3):953–958. doi: 10.1084/jem.171.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Winkelstein A. Mechanisms of immunosuppression: effects of cyclophosphamide on cellular immunity. Blood. 1973 Feb;41(2):273–284. [PubMed] [Google Scholar]



