Abstract
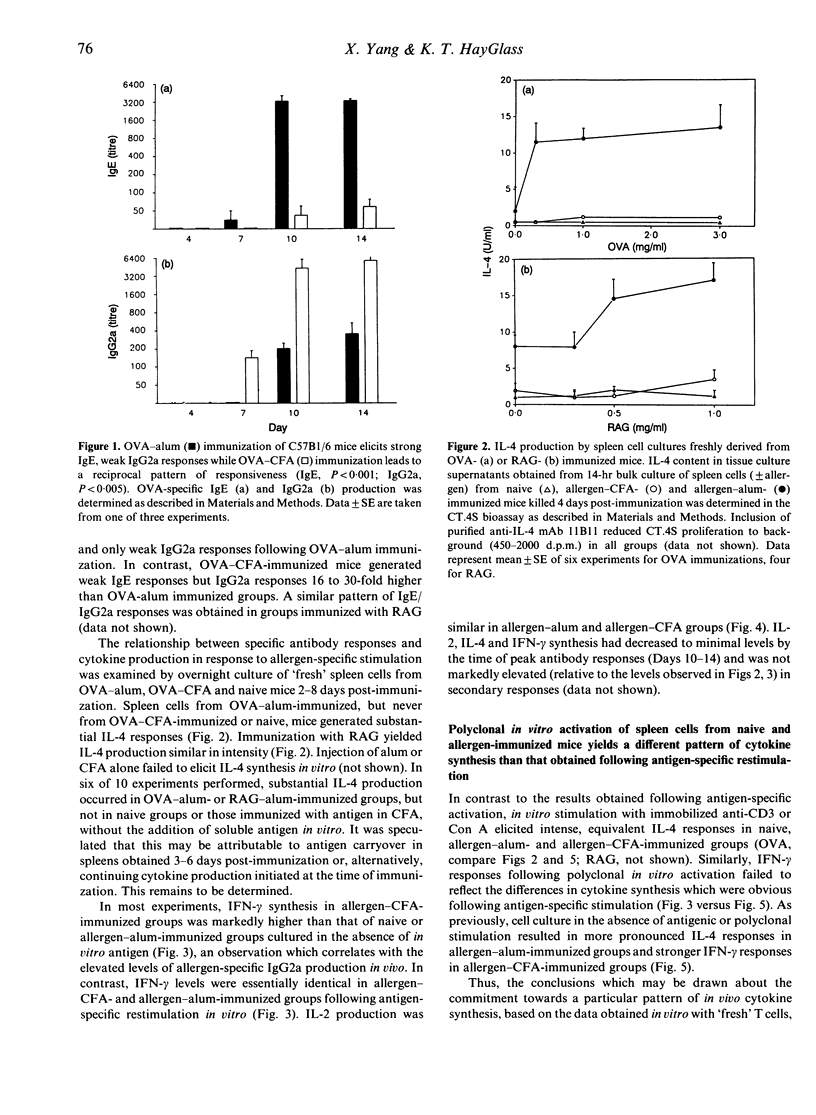
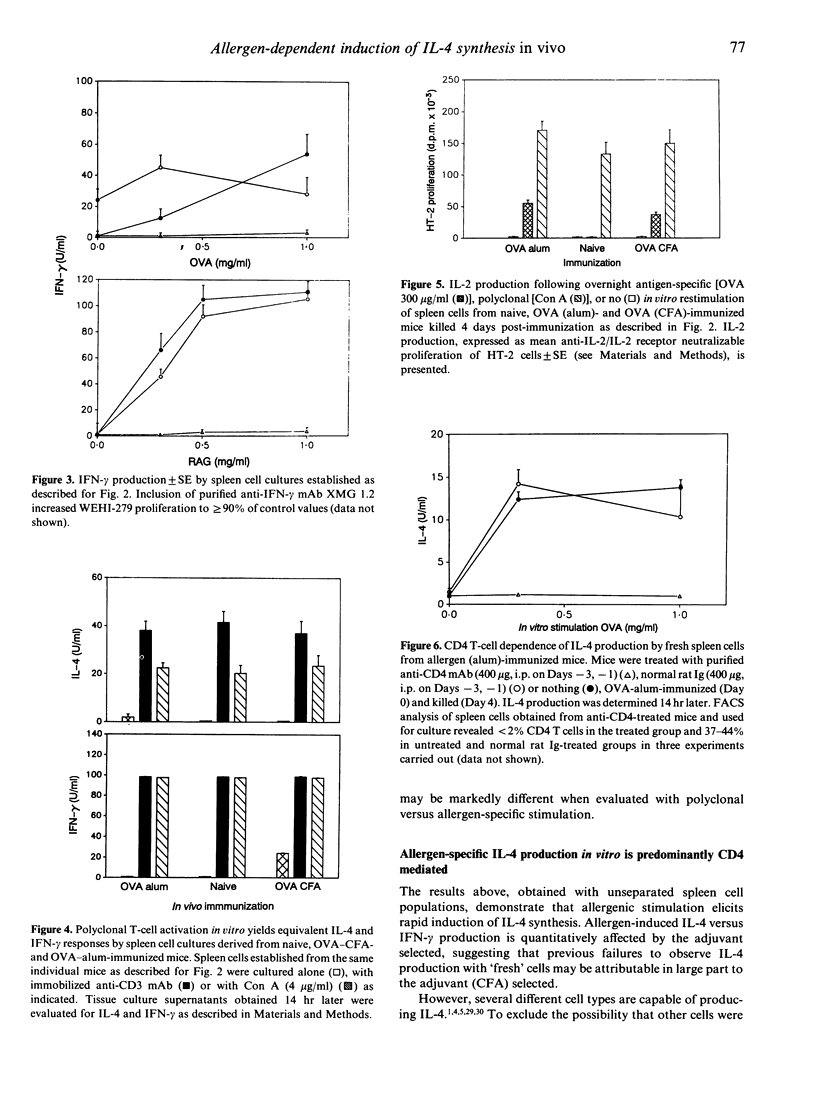
The ability of freshly derived T cells to produce interleukin-4 (IL-4) remains controversial. Many groups report that freshly derived antigen-, allogen- or mitogen-activated CD4 T cells produce almost exclusively IL-2, acquiring the capacity to produce a spectrum of cytokines following culture, rest and restimulation. In contrast, it is demonstrated here that in vivo exposure to protein allergens induces rapid, co-ordinate activation of IL-4 and interferon-gamma (IFN-gamma) gene expression. Overnight culture of spleen cells obtained from mice immunized with ovalbumin (OVA) or ragweed extract (RAG) in alum adjuvant elicits strong IL-2 and IL-4 responses within 14 hr of culture. T cells from mice immunized with allergen in complete Freund's adjuvant (CFA) generate strong IL-2 and IFN-gamma production, but virtually no IL-4, while unimmunized mice do not respond detectably to allergen in vitro (< 1 U). Unlike the differential pattern of cytokine synthesis observed following antigen-specific in vitro restimulation, cultures derived from naive and allergen-primed animals yield virtually equivalent IL-4, IL-2 and IFN-gamma synthesis upon polyclonal restimulation with anti-CD3 mAb 145-2C11 or concanavalin A (Con A), a finding which underlines the importance of the experimental conditions selected for in vitro analysis of in vivo cytokine gene expression. Collectively, the results indicate that the mode of immunization is critical to the pattern of cytokine response elicited and consequently to the type of antibody response which develops.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990 Aug 15;145(4):1127–1136. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Tonkonogy S., Swain S. L. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991 Sep 1;174(3):547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Flamand V., Abramowicz D., Goldman M., Biernaux C., Huez G., Urbain J., Moser M., Leo O. Anti-CD3 antibodies induce T cells from unprimed animals to secrete IL-4 both in vitro and in vivo. J Immunol. 1990 Apr 15;144(8):2875–2882. [PubMed] [Google Scholar]
- Fox B. S., Dordai D., Moore R. L., Lacy B. E. In vivo priming of helper T cells in the absence of B cell activation. J Immunol. 1989 Dec 15;143(12):3887–3893. [PubMed] [Google Scholar]
- HayGlass K. T., Naides S. J., Benacerraf B., Sy M. S. T cell development in B cell deficient mice. III. Restriction specificity of suppressor T cell factor(s) produced in mice treated chronically with rabbit anti-mouse mu chain antibody. J Mol Cell Immunol. 1985;2(2):107–117. [PubMed] [Google Scholar]
- HayGlass K. T., Stefura B. P. Anti-interferon gamma treatment blocks the ability of glutaraldehyde-polymerized allergens to inhibit specific IgE responses. J Exp Med. 1991 Feb 1;173(2):279–285. doi: 10.1084/jem.173.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Phenotypic and functional alteration of CD4+ T cells after antigen stimulation. Resolution of two populations of memory T cells that both secrete interleukin 4. J Exp Med. 1989 Jun 1;169(6):2245–2250. doi: 10.1084/jem.169.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Ishizaka K. Regulation of immunoglobin E biosynthesis. Adv Immunol. 1989;47:1–44. [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Kang S. S., Paul W. E., Finkelman F. D. IL-4 requirements for the generation of secondary in vivo IgE responses. J Immunol. 1991 Jun 15;146(12):4215–4221. [PubMed] [Google Scholar]
- Kishimoto T. IgE class-specific suppressor T cells and regulation of the IgE response. Prog Allergy. 1982;32:265–317. [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Conrad D. H., Clark-Lewis I., Finkelman F. D., Plaut M., Paul W. E. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol. 1990 Oct 15;145(8):2500–2506. [PubMed] [Google Scholar]
- Lord P. C., Wilmoth L. M., Mizel S. B., McCall C. E. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest. 1991 Apr;87(4):1312–1321. doi: 10.1172/JCI115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Differential production of IL-2 and IL-4 mRNA in vivo after primary sensitization. J Immunol. 1990 Sep 15;145(6):1734–1739. [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Powell T. J., Jr, Streilein J. W. Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990 Feb 1;144(3):854–859. [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Puré E., Inaba K., Metlay J. Lymphokine production by murine T cells in the mixed leukocyte reaction. J Exp Med. 1988 Aug 1;168(2):795–800. doi: 10.1084/jem.168.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Röcken M., Müller K. M., Saurat J. H., Hauser C. Lectin-mediated induction of IL-4-producing CD4+ T cells. J Immunol. 1991 Jan 15;146(2):577–584. [PubMed] [Google Scholar]
- Scott D. E., Gause W. C., Finkelman F. D., Steinberg A. D. Anti-CD3 antibody induces rapid expression of cytokine genes in vivo. J Immunol. 1990 Oct 1;145(7):2183–2188. [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Seder R. A., Le Gros G., Ben-Sasson S. Z., Urban J., Jr, Finkelman F. D., Paul W. E. Increased frequency of interleukin 4-producing T cells as a result of polyclonal priming. Use of a single-cell assay to detect interleukin 4-producing cells. Eur J Immunol. 1991 May;21(5):1241–1247. doi: 10.1002/eji.1830210522. [DOI] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Weinberg A. D., Hancock W. Characterization of T helper 1 and 2 cell subsets in normal mice. Helper T cells responsible for IL-4 and IL-5 production are present as precursors that require priming before they develop into lymphokine-secreting cells. J Immunol. 1988 Nov 15;141(10):3445–3455. [PubMed] [Google Scholar]
- Wang H., Mohapatra S. S., HayGlass K. T. Evidence for the existence of IL-4 and IFN gamma secreting cells in the T cell repertoire of naive mice. Immunol Lett. 1992 Feb;31(2):169–175. doi: 10.1016/0165-2478(92)90142-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. D., English M., Swain S. L. Distinct regulation of lymphokine production is found in fresh versus in vitro primed murine helper T cells. J Immunol. 1990 Mar 1;144(5):1800–1807. [PubMed] [Google Scholar]



