Abstract
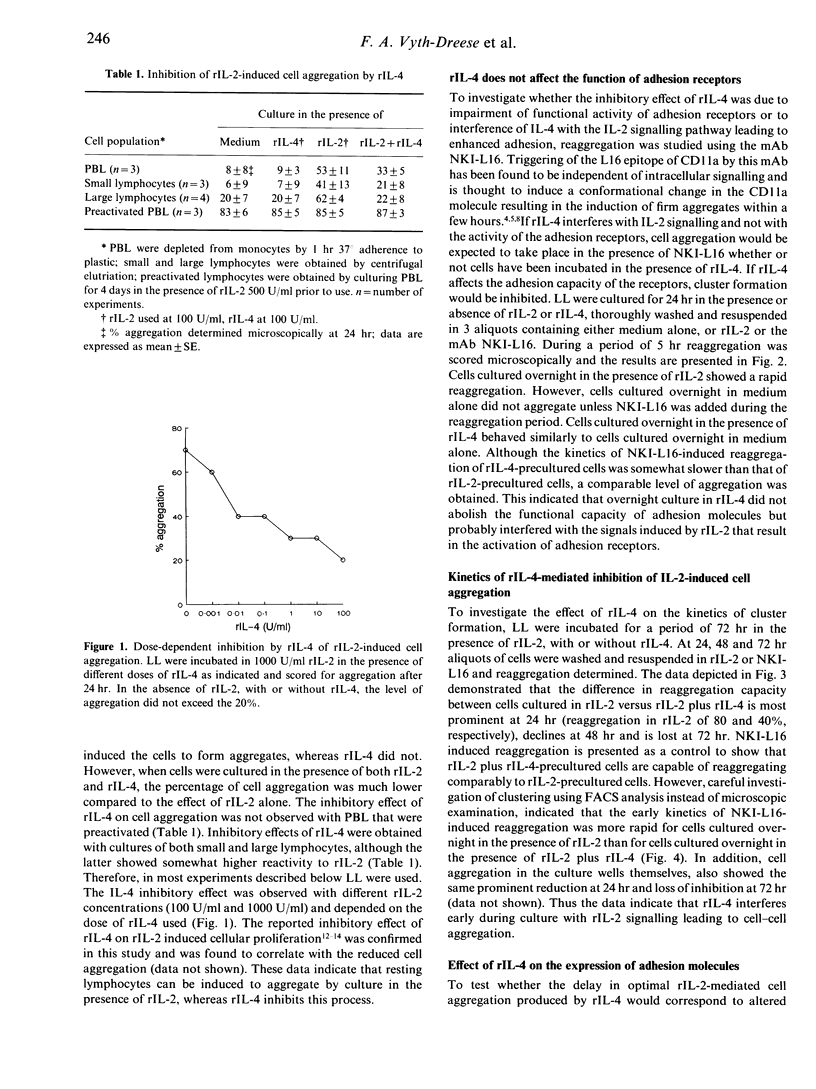
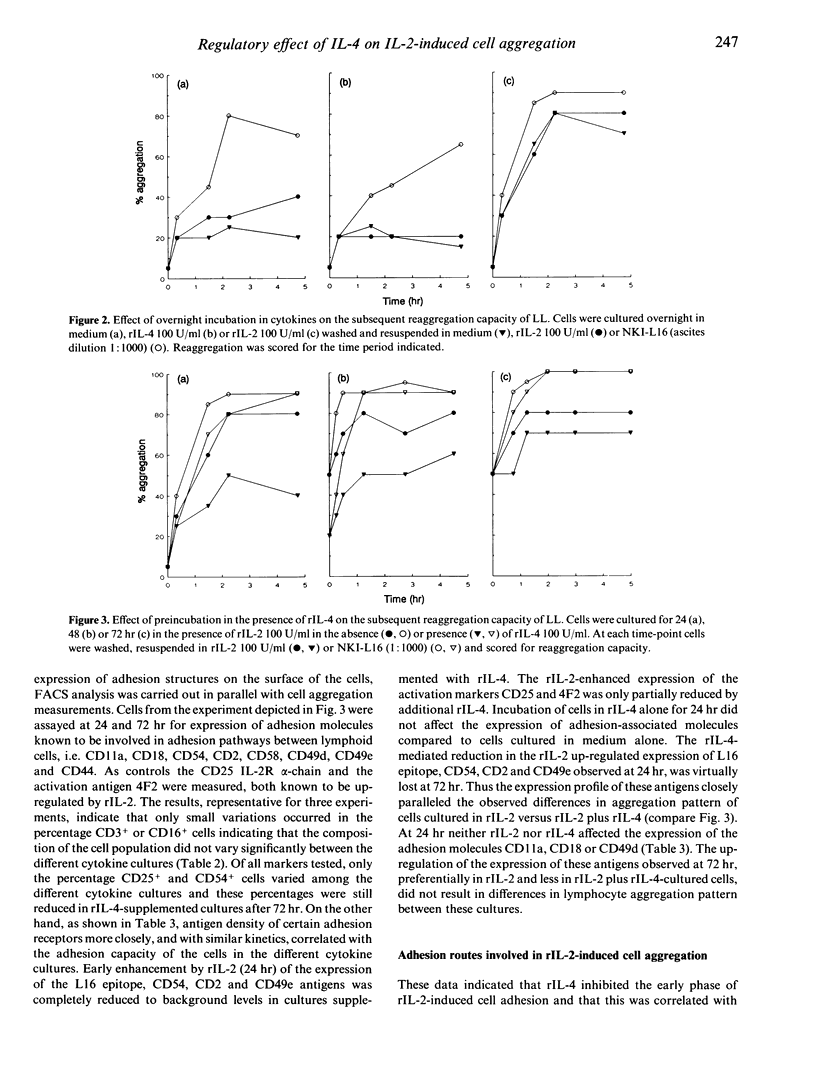
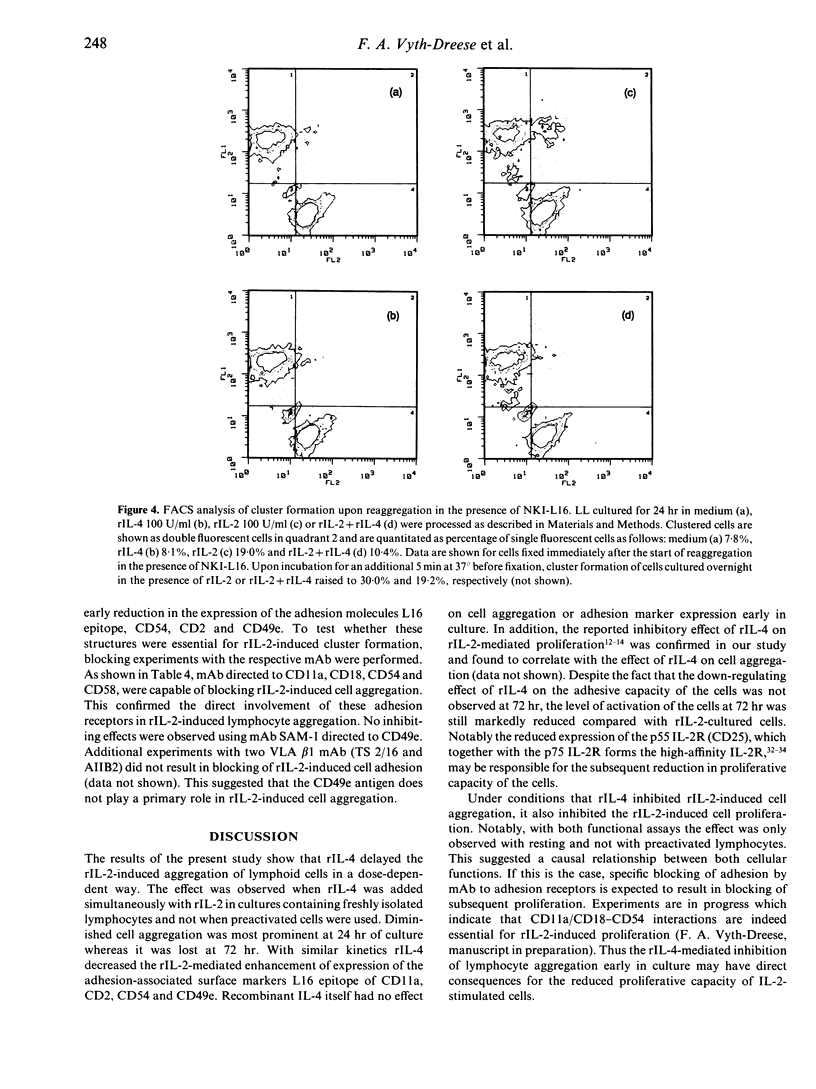
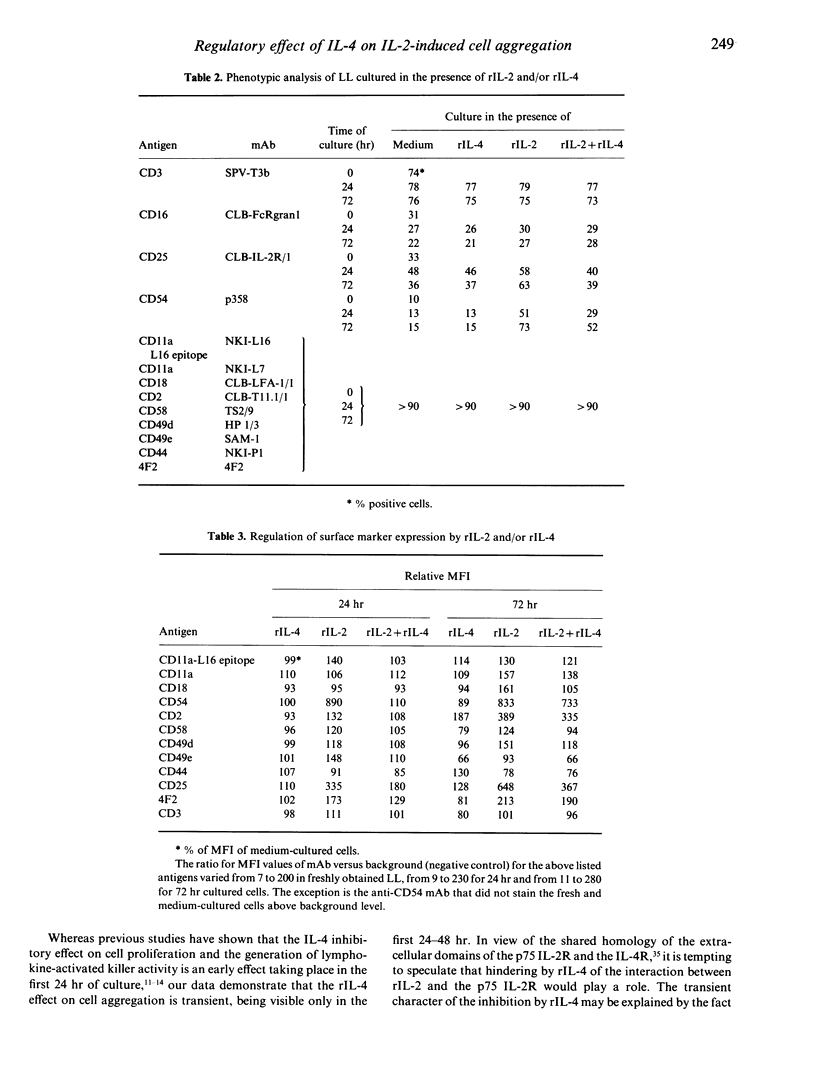
Human recombinant interleukin-4 (rIL-4) was studied for its capacity to inhibit rIL-2-induced lymphoid cell aggregation. In contrast to rIL-2, rIL-4 was unable to induce cluster formation by itself. However, when added simultaneously with rIL-2 to cultures of freshly isolated peripheral blood lymphocytes (PBL), rIL-4 inhibited cell aggregation in a dose-dependent way. In contrast, PBL, preactivated by a 4-day culture in the presence of 500 U/ml rIL-2, were not inhibited in their adhesive capacity by rIL-4. Inhibition of cell aggregation was most prominent at 24 hr and virtually lost after 72 hr of culture. Phenotypical analysis revealed that rIL-4, with similar kinetics, decreased the rIL-2-mediated up-regulation of the CD2, CD54 and CD49e adhesion molecules. In addition, it was observed that up-regulation of the activation epitope on CD11a recognized by the mAb NKI-L16, was prevented. During 24hr of culture rIL-4 itself did not alter the expression of these antigens. Blocking experiments with mAb directed against adhesion structures did not reveal a direct role for CD49e, but obviously demonstrated involvement of CD11a/CD18-CD54 and CD2-CD58 interactions in the rIL-2-induced adhesion. Therefore, rIL-4 appears to inhibit the early phase of rIL-2-induced aggregation by preventing the up-regulation of CD54 and CD2 antigens and by inhibiting the generation of the activated state of the CD11a/CD18 receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. Haemopoietic receptors and helical cytokines. Immunol Today. 1990 Oct;11(10):350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Bloemen P., Moldenhauer G., van Dijk M., Schuurman H. J., Bloem A. C. Multiple ICAM-1 (CD54) epitopes are involved in homotypic B-cell adhesion. Scand J Immunol. 1992 May;35(5):517–523. doi: 10.1111/j.1365-3083.1992.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Buckle A. M., Hogg N. Human memory T cells express intercellular adhesion molecule-1 which can be increased by interleukin 2 and interferon-gamma. Eur J Immunol. 1990 Feb;20(2):337–341. doi: 10.1002/eji.1830200216. [DOI] [PubMed] [Google Scholar]
- Campanero M. R., Pulido R., Ursa M. A., Rodríguez-Moya M., de Landázuri M. O., Sánchez-Madrid F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J Cell Biol. 1990 Jun;110(6):2157–2165. doi: 10.1083/jcb.110.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Figdor C. G., Bont W. S., De Vries J. E., Van Es W. L. Isolation of large numbers of highly purified lymphocytes and monocytes with a modified centrifugal elutriation technique. J Immunol Methods. 1981;40(3):275–288. doi: 10.1016/0022-1759(81)90359-8. [DOI] [PubMed] [Google Scholar]
- Figdor C. G., van Kooyk Y., Keizer G. D. On the mode of action of LFA-1. Immunol Today. 1990 Aug;11(8):277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Stade B. G., Hupke U., Holzmann B., Riethmüller G. The melanoma progression-associated antigen P3.58 is identical to the intercellular adhesion molecule, ICAM-1. Immunobiology. 1988 Dec;178(3):275–284. doi: 10.1016/S0171-2985(88)80071-8. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Custer M. C., Rosenberg S. A., Lotze M. T. IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol. 1989 May 15;142(10):3452–3461. [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Visser W., Vliem M., Figdor C. G. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988 Mar 1;140(5):1393–1400. [PubMed] [Google Scholar]
- Kuypers T. W., Koenderman L., Weening R. S., Verhoeven A. J., Roos D. Continuous cell activation is necessary for stable interaction of complement receptor type 3 with its counter-structure in the aggregation response of human neutrophils. Eur J Immunol. 1990 Mar;20(3):501–508. doi: 10.1002/eji.1830200307. [DOI] [PubMed] [Google Scholar]
- Martinez O. M., Gibbons R. S., Garovoy M. R., Aronson F. R. IL-4 inhibits IL-2 receptor expression and IL-2-dependent proliferation of human T cells. J Immunol. 1990 Mar 15;144(6):2211–2215. [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Hesselink W. G., Werner G., Spits H., Melief C. J. Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol. 1984 Jun;14(6):518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. The effects of IL-4 on human natural killer cells. A potent regulator of IL-2 activation and proliferation. J Immunol. 1988 Oct 1;141(7):2349–2351. [PubMed] [Google Scholar]
- Pals S. T., Hogervorst F., Keizer G. D., Thepen T., Horst E., Figdor C. C. Identification of a widely distributed 90-kDa glycoprotein that is homologous to the Hermes-1 human lymphocyte homing receptor. J Immunol. 1989 Aug 1;143(3):851–857. [PubMed] [Google Scholar]
- Park L. S., Friend D., Sassenfeld H. M., Urdal D. L. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987 Aug 1;166(2):476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Spits H., Keizer G., Borst J., Terhorst C., Hekman A., de Vries J. E. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2(4):423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- Spits H., Yssel H., Paliard X., Kastelein R., Figdor C., de Vries J. E. IL-4 inhibits IL-2-mediated induction of human lymphokine-activated killer cells, but not the generation of antigen-specific cytotoxic T lymphocytes in mixed leukocyte cultures. J Immunol. 1988 Jul 1;141(1):29–36. [PubMed] [Google Scholar]
- Spits H., van Schooten W., Keizer H., van Seventer G., van de Rijn M., Terhorst C., de Vries J. E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986 Apr 18;232(4748):403–405. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lier R. A., Brouwer M., Aarden L. A. Signals involved in T cell activation. T cell proliferation induced through the synergistic action of anti-CD28 and anti-CD2 monoclonal antibodies. Eur J Immunol. 1988 Jan;18(1):167–172. doi: 10.1002/eji.1830180125. [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese F. A., van der Reijden H. J., de Vries J. E. Phorbol-ester-mediated induction and augmentation of mitogenesis and interleukin-2 production in human T-cell lymphoproliferative disease. Blood. 1982 Dec;60(6):1437–1446. [PubMed] [Google Scholar]
- van Kooyk Y., Weder P., Hogervorst F., Verhoeven A. J., van Seventer G., te Velde A. A., Borst J., Keizer G. D., Figdor C. G. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991 Jan;112(2):345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van Kemenade P., Weder P., Kuijpers T. W., Figdor C. G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989 Dec 14;342(6251):811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- van Lier R. A., Boot J. H., Verhoeven A. J., de Groot E. R., Brouwer M., Aarden L. A. Functional studies with anti-CD3 heavy chain isotype switch-variant monoclonal antibodies. Accessory cell-independent induction of interleukin 2 responsiveness in T cells by epsilon-anti-CD3. J Immunol. 1987 Nov 1;139(9):2873–2879. [PubMed] [Google Scholar]


