Abstract
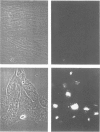
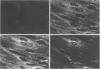
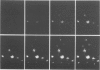
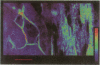
The effect of cytomegalovirus (CMV) infection on the cell surface expression of the adhesion molecules LFA-3 and ICAM-1 was studied by flow cytometry using human embryo lung fibroblasts. Our results demonstrated a marked increase in the expression of these molecules from days 1 to 5 post-infection. Peak expression of LFA-3 and ICAM-1 on the surface of the infected cell occurred at 2 days post-infection, when LFA-3 was twofold, and ICAM-1 threefold, greater than the level observed on uninfected fibroblasts. In contrast, parallel studies on the expression of class I HLA confirmed our previous findings that CMV induces a down-regulation of this molecule on the surface of the infected cell, and further demonstrated that at the time of maximum increase in LFA-3 and ICAM-1 expression, class I HLA expression was only 46% of the uninfected cell level. Immunofluorescence and confocal scanning laser microscopy revealed markedly enhanced expression of LFA-3 in infected cells, with accumulations in discrete granules in the perinuclear area, contrasting with the diffuse cytoplasmic distribution of this molecule in uninfected fibroblasts. ICAM-1 was found to be highly localized at the cell membrane of infected cells, whereas a diffuse cytoplasmic distribution was observed in the uninfected cell. The mechanism of the up-regulation of adhesion molecule expression on the CMV-infected cells remains to be determined; however cytokines known to up-regulate ICAM-1 were not detected in the culture supernatant of infected cells. The effects of CMV infection on the adhesion of peripheral blood leucocytes to fibroblasts is described in the accompanying manuscript (p. 413).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoni M., Faircloth M., Vugler L., Britt W. J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989 Feb;23(2):157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Barnes P. D., Grundy J. E. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J Gen Virol. 1992 Sep;73(Pt 9):2395–2403. doi: 10.1099/0022-1317-73-9-2395. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Rodgers B., Morris S., Graham S., Sissons J. G. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985 Apr;134(4):2695–2701. [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Detmers P. A., Wright S. D. Adhesion-promoting receptors on leukocytes. Curr Opin Immunol. 1988 Sep-Oct;1(1):10–15. doi: 10.1016/0952-7915(88)90045-3. [DOI] [PubMed] [Google Scholar]
- Duncombe A. S., Meager A., Prentice H. G., Grundy J. E., Heslop H. E., Hoffbrand A. V., Brenner M. K. Gamma-interferon and tumor necrosis factor production after bone marrow transplantation is augmented by exposure to marrow fibroblasts infected with cytomegalovirus. Blood. 1990 Sep 1;76(5):1046–1053. [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Griffiths P. D., Grundy J. E. Molecular biology and immunology of cytomegalovirus. Biochem J. 1987 Jan 15;241(2):313–324. doi: 10.1042/bj2410313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E. Alterations of cellular proteins in human cytomegalovirus infection: potential for disease pathogenesis. Transplant Proc. 1991 Jun;23(3 Suppl 3):38–42. [PubMed] [Google Scholar]
- Grundy J. E., Ayles H. M., McKeating J. A., Butcher R. G., Griffiths P. D., Poulter L. W. Enhancement of class I HLA antigen expression by cytomegalovirus: role in amplification of virus infection. J Med Virol. 1988 Aug;25(4):483–495. doi: 10.1002/jmv.1890250412. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Pahal G. S., Akbar A. N. Increased adherence of CD2 peripheral blood lymphocytes to cytomegalovirus-infected fibroblasts is blocked by anti-LFA-3 antibody. Immunology. 1993 Mar;78(3):413–420. [PMC free article] [PubMed] [Google Scholar]
- Hogg N. Roll, roll, roll your leucocyte gently down the vein.... Immunol Today. 1992 Apr;13(4):113–115. doi: 10.1016/0167-5699(92)90103-E. [DOI] [PubMed] [Google Scholar]
- Piela-Smith T. H., Broketa G., Hand A., Korn J. H. Regulation of ICAM-1 expression and function in human dermal fibroblasts by IL-4. J Immunol. 1992 Mar 1;148(5):1375–1381. [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. S., Grundy J. E. Beta 2 microglobulin on the envelope of urinary cytomegalovirus is not associated with host class I human leukocyte antigen alpha chain. J Gen Virol. 1992 Mar;73(Pt 3):507–512. doi: 10.1099/0022-1317-73-3-507. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span A. H., Mullers W., Miltenburg A. M., Bruggeman C. A. Cytomegalovirus induced PMN adherence in relation to an ELAM-1 antigen present on infected endothelial cell monolayers. Immunology. 1991 Mar;72(3):355–360. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]