Abstract
Soluble CD23 (sCD23) is increased by interleukin-4 (IL-4) and decreased by interferon-gamma (IFN-gamma). On the basis of cytokine profiles T-helper (Th) cells may be functionally divided into IL-2- and IFN-gamma-secreting Th1 cells, which are involved in cell-mediated immunity (CMI), and IL-4- and IL-5-producing Th2 cells, which are involved in humoral immunity. Compared with sex-matched controls (median 8.5) we found significantly elevated levels of serum sCD23 in patients with rheumatoid arthritis (median 22.7, P < 0.0002), with the highest levels detected in patients fulfilling an increasing number of the American Association revised criteria for rheumatoid arthritis. Soluble CD23 levels were also significantly raised in autoimmune thyroiditis (median 11.7, P < 0.02) and myasthenia gravis (median 10.4, P < 0.05). In contrast patients with either coeliac (median 6.5) or Crohn's disease (median 5.8) had reduced levels of sCD23 compared to appropriate controls (median 11.8), in both cases significant at P < 0.01. Variations in sCD23 may, therefore, reflect enhanced Th1 activity in the two later conditions in contrast to heightened Th2 activity within the three classical autoimmune conditions.
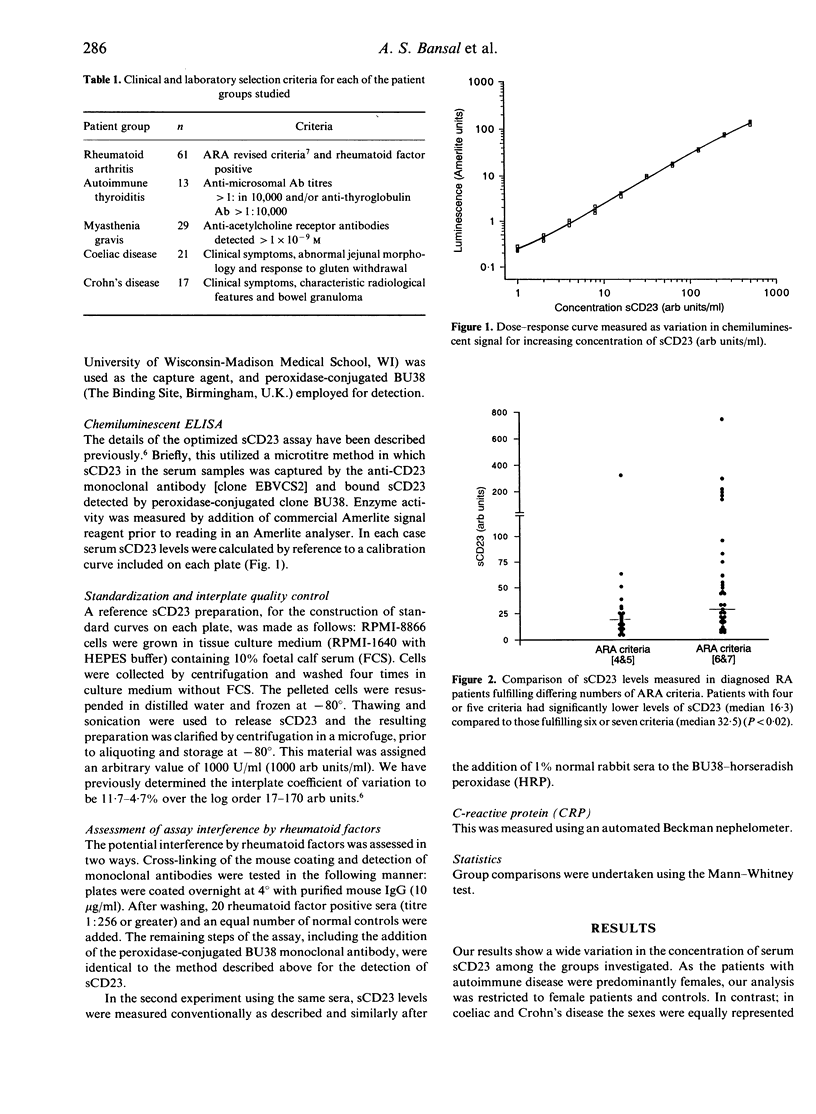
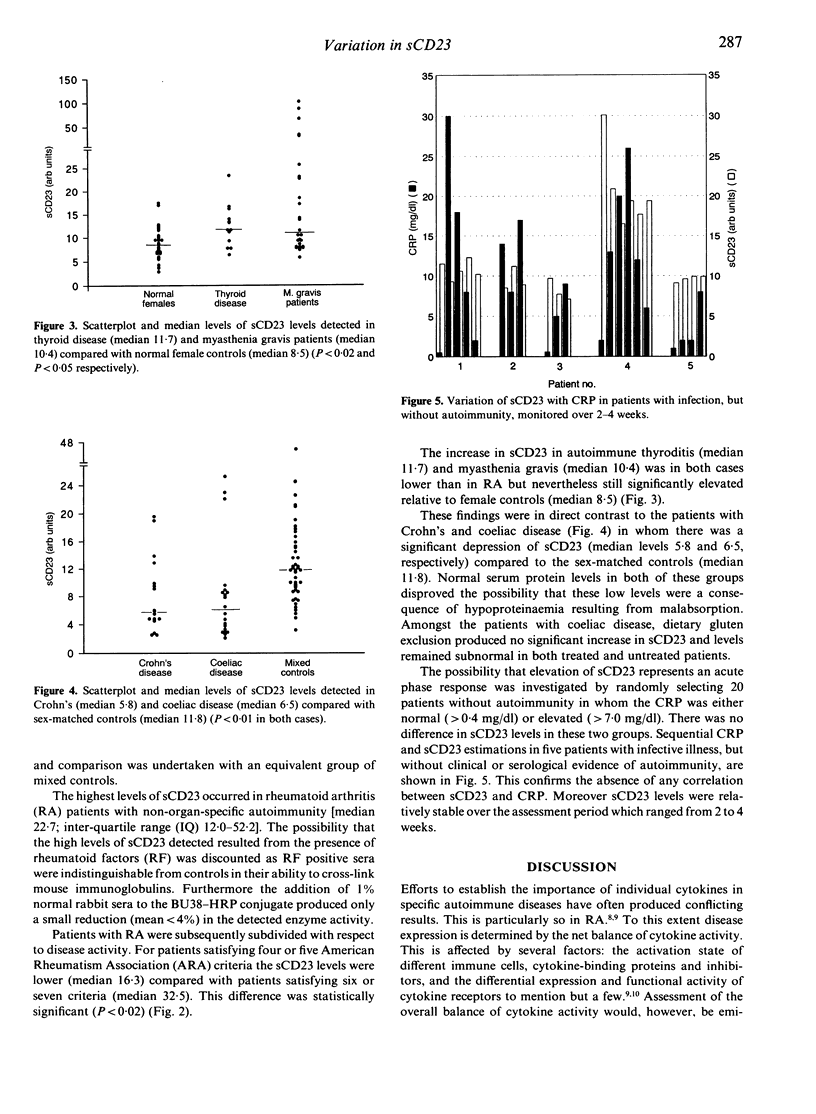
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Aubry J. P., Pochon S., Graber P., Jansen K. U., Bonnefoy J. Y. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992 Aug 6;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- Bansal A., Roberts T., Hay E. M., Kay R., Pumphrey R. S., Wilson P. B. Soluble CD23 levels are elevated in the serum of patients with primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 1992 Sep;89(3):452–455. doi: 10.1111/j.1365-2249.1992.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Guillot O., Spits H., Blanchard D., Ishizaka K., Banchereau J. The low-affinity receptor for IgE (CD23) on B lymphocytes is spatially associated with HLA-DR antigens. J Exp Med. 1988 Jan 1;167(1):57–72. doi: 10.1084/jem.167.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespesse G., Suter U., Mossalayi D., Bettler B., Sarfati M., Hofstetter H., Kilcherr E., Debre P., Dalloul A. Expression, structure, and function of the CD23 antigen. Adv Immunol. 1991;49:149–191. doi: 10.1016/s0065-2776(08)60776-2. [DOI] [PubMed] [Google Scholar]
- Goldman M., Druet P., Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991 Jul;12(7):223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- Gordon J. CD23 and B cell activation. Clin Exp Allergy. 1992 Feb;22(2):199–204. doi: 10.1111/j.1365-2222.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Mossalayi M. D., Dalloul A. H., Fourcade C., Arock M., Merle-Beral H., Hofstetter H., Debré P. The role of CD23 on early human hematopoietic precursors. Monogr Allergy. 1991;29:178–185. [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Ruschen S., Stellberg W., Warnatz H. Kinetics of cytokine secretion by mononuclear cells of the blood from rheumatoid arthritis patients are different from those of healthy controls. Clin Exp Immunol. 1992 Jul;89(1):32–37. doi: 10.1111/j.1365-2249.1992.tb06873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Bettler B., Letellier M., Fournier S., Rubio-Trujillo M., Hofstetter H., Delespesse G. Native and recombinant soluble CD23 fragments with IgE suppressive activity. Immunology. 1992 Aug;76(4):662–667. [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W. The spectrum of action of new immunosuppressive drugs. Clin Exp Immunol. 1992 Aug;89(2):170–173. doi: 10.1111/j.1365-2249.1992.tb06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



